- Record: found
- Abstract: found
- Article: found
Gut-associated lymphoid tissue: a microbiota-driven hub of B cell immunity
Read this article at
Highlights
-
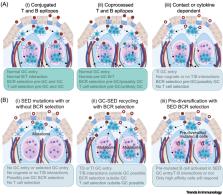
Mammalian gut-associated lymphoid tissue (GALT) is chronically activated by the intestinal microbiota throughout life.
-
GALT propagates and selects B cells in germinal centers, including B cells recognizing T-cell-independent carbohydrate antigens.
-
GALT supports the development of systemic B cells in the so-called GALT species, including humans.
-
In humans, GALT supports the development of innate-like marginal zone B cells that circulate in blood, mostly reside in the spleen, and can protect the lungs.
Significance
Gut-associated lymphoid tissue (GALT) is located throughout the gastrointestinal tract and is indispensable for the maintenance of gut homeostasis and human health. Recent advances increase our understanding of B cell responses to microbiota in GALT that can generate antibodies to antigens often shared by microbiota species, including T cell independent, yet germinal-center-matured responses. Indeed, chronic stimulation of GALT B cells generates a niche for B cell maturation and propagation in some species, including marginal zone B cells in humans.
Abstract
The diverse gut microbiota, which is associated with mucosal health and general wellbeing, maintains gut-associated lymphoid tissues (GALT) in a chronically activated state, including sustainment of germinal centers in a context of high antigenic load. This influences the rules for B cell engagement with antigen and the potential consequences. Recent data have highlighted differences between GALT and other lymphoid tissues. For example, GALT propagates IgA responses against glycans that show signs of having been generated in germinal centers. Other findings suggest that humans are among those species where GALT supports the diversification, propagation, and possibly selection of systemic B cells. Here, we review novel findings that identify GALT as distinctive, and able to support these processes.
Abstract
The diverse gut microbiota, which is associated with mucosal health and general wellbeing, maintains gut-associated lymphoid tissues (GALT) in a chronically activated state, including sustainment of germinal centers in a context of high antigenic load. This influences the rules for B cell engagement with antigen and the potential consequences. Recent data have highlighted differences between GALT and other lymphoid tissues. For example, GALT propagates IgA responses against glycans that show signs of having been generated in germinal centers. Other findings suggest that humans are among those species where GALT supports the diversification, propagation, and possibly selection of systemic B cells. Here, we review novel findings that identify GALT as distinctive, and able to support these processes.
Related collections
Most cited references84
- Record: found
- Abstract: found
- Article: not found
Visualization and analysis of gene expression in tissue sections by spatial transcriptomics.
- Record: found
- Abstract: found
- Article: not found
Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus
- Record: found
- Abstract: found
- Article: not found