- Record: found
- Abstract: found
- Article: found
A pilot open label, single dose trial of fenobam in adults with fragile X syndrome
Read this article at
Abstract
Objective:
A pilot open label, single dose trial of fenobam, an mGluR5 antagonist, was conducted to provide an initial evaluation of safety and pharmacokinetics in adult males and females with fragile X syndrome (FXS).
Methods:
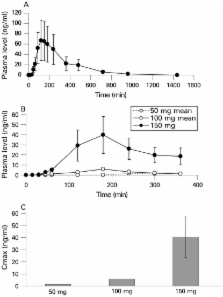
Twelve subjects, recruited from two fragile X clinics, received a single oral dose of 50–150 mg of fenobam. Blood for pharmacokinetic testing, vital signs and side effect screening was obtained at baseline and numerous time points for 6 h after dosing. Outcome measures included prepulse inhibition (PPI) and a continuous performance test (CPT) obtained before and after dosing to explore the effects of fenobam on core phenotypic measures of sensory gating, attention and inhibition.
Results:
There were no significant adverse reactions to fenobam administration. Pharmacokinetic analysis showed that fenobam concentrations were dose dependent but variable, with mean (SEM) peak values of 39.7 (18.4) ng/ml at 180 min after the 150 mg dose. PPI met a response criterion of an improvement of at least 20% over baseline in 6 of 12 individuals (4/6 males and 2/6 females). The CPT did not display improvement with treatment due to ceiling effects.
Conclusions:
Clinically significant adverse effects were not identified in this study of single dose fenobam across the range of dosages utilised. The positive effects seen in animal models of FXS treated with fenobam or other mGluR5 antagonists, the apparent lack of clinically significant adverse effects, and the potential beneficial clinical effects seen in this pilot trial support further study of the compound in adults with FXS.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome.
- Record: found
- Abstract: found
- Article: not found
Correction of fragile X syndrome in mice.
- Record: found
- Abstract: found
- Article: not found