- Record: found
- Abstract: found
- Article: found
Overexpression of lncRNA EPB41L4A-AS1 Induces Metabolic Reprogramming in Trophoblast Cells and Placenta Tissue of Miscarriage
Read this article at
Abstract
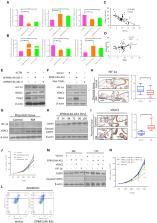
Long non-coding RNAs (lncRNAs) have been shown to be crucial regulators in numerous human diseases. However, little is known about their effects on early recurrent miscarriage (RM). Here we aimed to investigate the role of lncRNA EPB41L4A-AS1 on placental trophoblast cell metabolic reprogramming, which might be involved in the pathogenesis of RM. After microarray and GEO database analyses, we found that EPB41L4A-AS1 was significantly increased in early RM placental tissue, and this increase may relate to estradiol-mediated upregulation of PGC-1α. EPB41L4A-AS1 overexpression inhibits glycolysis but increases the dependence on fatty acid oxidation in mitochondrion metabolism and suppresses the Warburg effect, which is necessary for rapid growth of the placental villus, leading to miscarriage. Mechanistic analyses demonstrated that EPB41L4A-AS1 functions as a lncRNA in the regulation of VDAC1 and HIF-1α expression through enhancement of H3K4me3 levels in the promoters of VDAC1 and HIF1A-AS1, a natural antisense transcript (NAT) lncRNA of HIF-1α. Taken together, these findings demonstrate that aberrant expression of EPB41L4A-AS1 is involved in the etiology of early RM, and it may be a candidate diagnostic hallmark and a potential therapeutic target for early RM treatment.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
LncRNA HOXA11-AS Promotes Proliferation and Invasion of Gastric Cancer by Scaffolding the Chromatin Modification Factors PRC2, LSD1, and DNMT1.
- Record: found
- Abstract: found
- Article: not found
Endocrine disruptors: from endocrine to metabolic disruption.
- Record: found
- Abstract: found
- Article: found