- Record: found
- Abstract: found
- Article: found
DNA-methylation-mediated activating of lncRNA SNHG12 promotes temozolomide resistance in glioblastoma
Read this article at
Abstract
Background
Accumulating evidence shows that long noncoding RNAs (lncRNAs) are important regulator molecules involved in diverse biological processes. Acquired drug resistance is a major challenge in the clinical treatment of glioblastoma (GBM), and lncRNAs have been shown to play a role in chemotherapy resistance. However, the underlying mechanisms by which lncRNA mediates TMZ resistance in GBM remain poorly characterized.
Methods
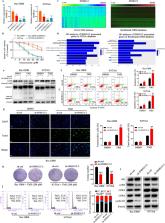
Quantitative reverse transcription PCR (qRT-PCR) and fluorescence in situ hybridization assays were used to detect small nucleolar RNA host gene 12 (SNHG12) levels in TMZ-sensitive and TMZ-resistant GBM cells and tissues. The effects of SNHG12 on TMZ resistance were investigated through in vitro assays (western blots, colony formation assays, flow cytometry assays, and TUNEL assays). The mechanism mediating the high expression of SNHG12 in TMZ-resistant cells and its relationships with miR-129-5p, mitogen-activated protein kinase 1 (MAPK1), and E2F transcription factor 7 (E2F7) were determined by bioinformatic analysis, bisulfite amplicon sequencing, methylation-specific PCR, dual luciferase reporter assays, chromatin immunoprecipitation assays, RNA immunoprecipitation assays, immunofluorescence, qRT-PCR, and western blot. For in vivo experiments, an intracranial xenograft tumor mouse model was used to investigate SNHG12 function.
Results
SNHG12 was upregulated in TMZ-resistant cells and tissues. Overexpression of SNHG12 led to the development of acquired TMZ resistance, while knockdown of SNHG12 restored TMZ sensitivity. An abnormally low level of DNA methylation was detected within the promoter region of SNHG12, and loss of DNA methylation made this region more accessible to the Sp1 transcription factor (SP1); this indicated that methylation and SP1 work together to regulate SNHG12 expression. In the cytoplasm, SNHG12 served as a sponge for miR-129-5p, leading to upregulation of MAPK1 and E2F7 and endowing the GBM cells with TMZ resistance. Disinhibition of MAPK1 regulated TMZ-induced cell apoptosis and the G1/S cell cycle transition by activating the MAPK/ERK pathway, while E2F7 dysregulation was primarily associated with G1/S cell cycle transition. Clinically, SNHG12 overexpression was associated with poor survival of GBM patients undergoing TMZ treatment.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
A restricted cell population propagates glioblastoma growth following chemotherapy
- Record: found
- Abstract: found
- Article: found
Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience
- Record: found
- Abstract: found
- Article: not found