- Record: found
- Abstract: found
- Article: found
Hepatic arterial infusion chemotherapy combined with anti-PD-1/PD-L1 immunotherapy and molecularly targeted agents for advanced hepatocellular carcinoma: a real world study
Read this article at
Abstract
Background
Molecular targeted therapy combined with immunotherapy significantly improves the prognosis of patients with advanced liver cancer. Additionally, hepatic arterial infusion chemotherapy (HAIC) can improve the prognosis of patients with advanced liver cancer. This real-world study aimed to evaluate the clinical efficacy and safety of HAIC combined with molecular targeted therapy and immunotherapy in the treatment of primary unresectable hepatocellular carcinoma (uHCC).
Methods
A total of 135 patients with uHCC were enrolled in this study. Progression-free survival (PFS) was the primary endpoint. The efficacy of the combination therapy was assessed based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST) guidelines. Overall survival (OS), adverse events (AEs) and surgical conversion rate were the secondary endpoints. Univariate and multivariate Cox regression analyses were performed to examine independent prognostic factors. For sensitivity analysis, inverse probability weighting (IPW) was used to balance the influence of the tested confounding factors between groups to verify the robustness of conversion surgery for survival benefits. The E-values were estimated to assess robustness to unmeasured confounders.
Results
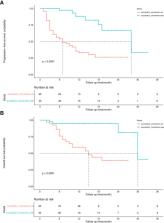
The median number of therapies was three. Approximately 60% of the patients had portal vein tumour thrombosis (PVTT). The most common targeted drugs were lenvatinib and bevacizumab, whereas the most common immunotherapy drug was sintilimab. The overall objective response rate (ORR) was 54.1%, and the disease control rate (DCR) was 94.6%. A total of 97 (72%) patients experienced AEs of grades 3–4. Fatigue, pain and fever were the most common symptoms of grade 3-4 AEs. The median PFS was 28 months and 7 months in the successful and unsuccessful conversion groups, respectively. The median OS was 30 months and 15 months in the successful and unsuccessful conversion groups, respectively. Successful conversion surgery, sex, hapatic vein invasion, BCLC stage, baseline tumour size, AFP levels and maximum therapeutic response were independent prognostic factors for PFS. Successful conversion surgery, number of interventions, hapatic vein invasion and total bilirubin levels were independent prognostic factors for OS. After IPTW, no standardised differences exceeding 0.1 were found. IPW-adjusted Kaplan–Meier curves showed that successful conversion surgery was an independent prognostic factor for both PFS and OS. The E-values of successful conversion surgery were 7.57 and 6.53 for OS and PFS, respectively, which indicated a relatively robust impact of successful conversion surgery on the prognosis of patients.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries
- Record: found
- Abstract: found
- Article: not found
Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma
- Record: found
- Abstract: found
- Article: not found