- Record: found
- Abstract: found
- Article: found
The Safety and Efficacy of Hepatic Arterial Infusion Chemotherapy Combined with PD-(L)1 Inhibitors and Molecular Targeted Therapies for the Treatment of Intermediate and Advanced Hepatocellular Carcinoma Unsuitable for Transarterial Chemoembolization
Read this article at
Abstract
Objective
To investigate the efficacy and safety of hepatic arterial infusion chemotherapy (HAIC) combined with PD-(L)1 inhibitors and molecular targeted therapies (MTT) for intermediate and advanced HCC that are unsuitable for transarterial chemoembolization (TACE).
Methods
We conducted a retrospective analysis of data from patients with TACE-unsuitable HCC who were receiving triple therapy from January 2020 to December 2021 at two medical centers. The primary outcome was overall survival (OS), and the secondary outcomes were progression-free survival (PFS), objective response rates (ORR), disease control rates (DCR), and incidence of adverse events (AEs).
Results
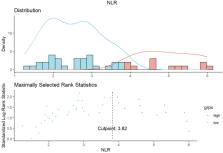
A total of 55 patients were enrolled in the study with median treatment periods of 4 and 6 for HAIC and PD-(L)1 inhibitors, respectively. The median OS and PFS were 15.0 and 10.0 months, respectively, with a median follow-up of 11.0 months (range: 4.0–27.5 months). According to the mRECIST criteria, the optimal ORR was 43.6% (24/55) and the DCR was 61.8% (34/55). The incidence of AEs was 58.2%, with grade 3 and above accounting for 20.0%; elevated AST (18.2%), hyperbilirubinemia (16.4%), and thrombocytopenia (16.4%) were most common. There were no treatment-related fatalities and all AEs were effectively managed. Multifactorial analysis showed that NLR > 3.82 (HR 2.380, 95% CI 1.116-2-5.079, P = 0.025), ECOG 1 (HR 2.906, 95% CI 1.373–6.154, P = 0.005), and extrahepatic metastases (HR 8.373, 95% CI 3.492–20.078, P < 0.001) were associated with the median OS.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries
- Record: found
- Abstract: found
- Article: not found
Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma
- Record: found
- Abstract: not found
- Article: not found