- Record: found
- Abstract: found
- Article: found
The BH3-only protein Bik/Blk/Nbk inhibits nuclear translocation of activated ERK1/2 to mediate IFNγ-induced cell death
Read this article at
Abstract
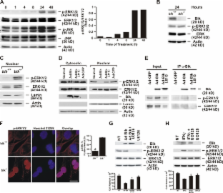
IFNγ induces cell death in epithelial cells, but the mediator for this death pathway has not been identified. In this study, we find that expression of Bik/Blk/Nbk is increased in human airway epithelial cells (AECs [HAECs]) in response to IFNγ. Expression of Bik but not mutant BikL61G induces and loss of Bik suppresses IFNγ-induced cell death in HAECs. IFNγ treatment and Bik expression increase cathepsin B and D messenger RNA levels and reduce levels of phospho–extracellular regulated kinase 1/2 (ERK1/2) in the nuclei of bik +/+ compared with bik −/− murine AECs. Bik but not BikL61G interacts with and suppresses nuclear translocation of phospho-ERK1/2, and suppression of ERK1/2 activation inhibits IFNγ- and Bik-induced cell death. Furthermore, after prolonged exposure to allergen, hyperplastic epithelial cells persist longer, and nuclear phospho-ERK is more prevalent in airways of IFNγ −/− or bik −/− compared with wild-type mice. These results demonstrate that IFNγ requires Bik to suppress nuclear localization of phospho-ERK1/2 to channel cell death in AECs.
Related collections
Most cited references62
- Record: found
- Abstract: found
- Article: not found
Endoplasmic reticulum stress: cell life and death decisions.
- Record: found
- Abstract: found
- Article: not found
Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis.
- Record: found
- Abstract: found
- Article: not found