- Record: found
- Abstract: found
- Article: found
Zerumbone-incorporated liquid crystalline nanoparticles inhibit proliferation and migration of non-small-cell lung cancer in vitro
Read this article at
Abstract
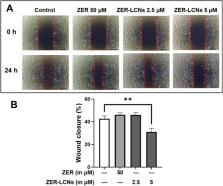
Lung cancer is the second most prevalent type of cancer and is responsible for the highest number of cancer-related deaths worldwide. Non-small-cell lung cancer (NSCLC) makes up the majority of lung cancer cases. Zerumbone (ZER) is natural compound commonly found in the roots of Zingiber zerumbet which has recently demonstrated anti-cancer activity in both in vitro and in vivo studies. Despite their medical benefits, ZER has low aqueous solubility, poor GI absorption and oral bioavailability that hinders its effectiveness. Liquid crystalline nanoparticles (LCNs) are novel drug delivery carrier that have tuneable characteristics to enhance and ease the delivery of bioactive compounds. This study aimed to formulate ZER-loaded LCNs and investigate their effectiveness against NSCLC in vitro using A549 lung cancer cells. ZER-LCNs, prepared in the study, inhibited the proliferation and migration of A549 cells. These inhibitory effects were superior to the effects of ZER alone at a concentration 10 times lower than that of free ZER, demonstrating a potent anti-cancer activity of ZER-LCNs. The underlying mechanisms of the anti-cancer effects by ZER-LCNs were associated with the transcriptional regulation of tumor suppressor genes P53 and PTEN, and metastasis-associated gene KRT18. The protein array data showed downregulation of several proliferation associated proteins such as AXL, HER1, PGRN, and BIRC5 and metastasis-associated proteins such as DKK1, CAPG, CTSS, CTSB, CTSD, and PLAU. This study provides evidence of potential for increasing the potency and effectiveness of ZER with LCN formulation and developing ZER-LCNs as a treatment strategy for mitigation and treatment of NSCLC.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries

- Record: found
- Abstract: found
- Article: found
Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems
- Record: found
- Abstract: found
- Article: not found