- Record: found
- Abstract: found
- Article: found
Cytomegalovirus (CMV) Pneumonitis: Cell Tropism, Inflammation, and Immunity
Read this article at
Abstract
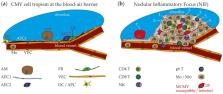
Human cytomegalovirus (HCMV) is an opportunistic pathogen causing disease mainly in immunocompromised patients or after congenital infection. HCMV infection of the respiratory tract leads to pneumonitis in the immunocompromised host, which is often associated with a bad clinical course. The related mouse cytomegalovirus (MCMV) likewise exhibits a distinct tropism for the lung and thus provides an elegant model to study host-pathogen interaction. Accordingly, fundamental features of cytomegalovirus (CMV) pneumonitis have been discovered in mice that correlate with clinical data obtained from humans. Recent studies have provided insight into MCMV cell tropism and localized inflammation after infection of the respiratory tract. Accordingly, the nodular inflammatory focus (NIF) has been identified as the anatomical correlate of immune control in lungs. Several hematopoietic cells involved in antiviral immunity reside in NIFs and their key effector molecules have been deciphered. Here, we review what has been learned from the mouse model with focus on the microanatomy of infection sites and antiviral immunity in MCMV pneumonitis.
Related collections
Most cited references75
- Record: found
- Abstract: found
- Article: found
Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review
- Record: found
- Abstract: found
- Article: not found
How we treat cytomegalovirus in hematopoietic cell transplant recipients.

- Record: found
- Abstract: found
- Article: found