- Record: found
- Abstract: found
- Article: found
Targeting the Tumor Extracellular Matrix by the Natural Molecule 4-Methylumbelliferone: A Complementary and Alternative Cancer Therapeutic Strategy
Read this article at
Abstract
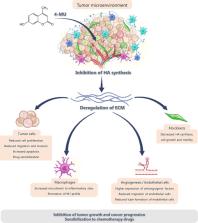
In antineoplastic therapy, one of the challenges is to adjust the treatment to the needs of each patient and reduce the toxicity caused by conventional antitumor strategies. It has been demonstrated that natural products with antitumoral properties are less toxic than chemotherapy and radiotherapy. Also, using already developed drugs allows developing substantially less costly methods for the discovery of new treatments than traditional drug development. Candidate molecules proposed for drug repositioning include 4-methylumbelliferone (4-MU), an orally available dietetic product, derivative of coumarin and mainly found in the plant family Umbelliferae or Apiaceae. 4-MU specifically inhibits the synthesis of glycosaminoglycan hyaluronan (HA), which is its main mechanism of action. This agent reduces the availability of HA substrates and inhibits the activity of different HA synthases. However, an effect independent of HA synthesis has also been observed. 4-MU acts as an inhibitor of tumor growth in different types of cancer. Particularly, 4-MU acts on the proliferation, migration and invasion abilities of tumor cells and inhibits the progression of cancer stem cells and the development of drug resistance. In addition, the effect of 4-MU impacts not only on tumor cells, but also on other components of the tumor microenvironment. Specifically, 4-MU can potentially act on immune, fibroblast and endothelial cells, and pro-tumor processes such as angiogenesis. Most of these effects are consistent with the altered functions of HA during tumor progression and can be interrupted by the action of 4-MU. While the potential advantage of 4-MU as an adjunct in cancer therapy could improve therapeutic efficacy and reduce toxicities of other antitumoral agents, the greatest challenge is the lack of scientific evidence to support its approval. Therefore, crucial human clinical studies have yet to be done to respond to this need. Here, we discuss and review the possible applications of 4-MU as an adjunct in conventional antineoplastic therapies, to achieve greater therapeutic success. We also describe the main proposed mechanisms of action that promote an increase in the efficacy of conventional antineoplastic strategies in different types of cancer and prospects that promote 4-MU repositioning and application in cancer therapy.
Related collections
Most cited references142
- Record: found
- Abstract: found
- Article: not found
Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019
- Record: found
- Abstract: found
- Article: not found
Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers.
- Record: found
- Abstract: found
- Article: not found