- Record: found
- Abstract: found
- Article: found
Unmasking a new prognostic marker and therapeutic target from the GDNF-RET/PIT1/p14ARF/p53 pathway in acromegaly
Read this article at
Abstract
Background
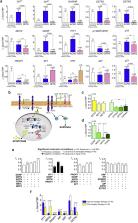
Acromegaly is produced by excess growth hormone secreted by a pituitary adenoma of somatotroph cells (ACRO). First-line therapy, surgery and adjuvant therapy with somatostatin analogs, fails in 25% of patients. There is no predictive factor of resistance to therapy. New therapies are investigated using few dispersed tumor cells in acute primary cultures in standard conditions where the cells do not grow, or using rat pituitary cell lines that do not maintain the full somatotroph phenotype. The RET/PIT1/p14ARF/p53 pathway regulates apoptosis in normal pituitary somatotrophs whereas the RET/GDNF pathway regulates survival, controlling PIT1 levels and blocking p14ARF (ARF) and p53 expression.
Methods
We investigated these two RET pathways in a prospective series of 32 ACRO and 63 non-functioning pituitary adenomas (NFPA), studying quantitative RNA and protein gene expression for molecular-clinical correlations and how the RET pathway might be implicated in therapeutic success. Clinical data was collected during post-surgical follow-up. We also established new'humanized’ pituitary cultures, allowing 20 repeated passages and maintaining the pituitary secretory phenotype, and tested five multikinase inhibitors (TKI: Vandetanib, Lenvatinib, Sunitinib, Cabozantinib and Sorafenib) potentially able to act on the GDNF-induced RET dimerization/survival pathway. Antibody arrays investigated intracellular molecular pathways.
Findings
In ACRO, there was specific enrichment of all genes in both RET pathways, especially GDNF. ARF and GFRA4 gene expression were found to be opposing predictors of response to first-line therapy. ARF cut-off levels, calculated categorizing by GNAS mutation, were predictive of good response (above) or resistance (below) to therapy months later. Sorafenib, through AMPK, blocked the GDNF/AKT survival action without altering the RET apoptotic pathway.
Interpretation
Tumor ARF mRNA expression measured at the time of the surgery is a prognosis factor in acromegaly. The RET inhibitor, Sorafenib, is proposed as a potential treatment for resistant ACRO.
Fund
This project was supported by national grants from Agencia Estatal de Investigación (AEI) and Instituto Investigación Carlos III, with participation of European FEDER funds, to IB (PI150056) and CVA (BFU2016-76973-R). It was also supported initially by a grant from the Investigator Initiated Research (IIR) Program (WI177773) and by a non-restricted Research Grant from Pfizer Foundation to IB. Some of the pituitary acromegaly samples were collected in the framework of the Spanish National Registry of Acromegaly (REMAH), partially supported by an unrestricted grant from Novartis to the Spanish Endocrine Association (SEEN).
CVA is also supported from a grant of Medical Research Council UK MR/M018539/1.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Acromegaly: an endocrine society clinical practice guideline.
- Record: found
- Abstract: found
- Article: not found
The prevalence of pituitary adenomas: a systematic review.
- Record: found
- Abstract: found
- Article: not found