- Record: found
- Abstract: found
- Article: found
Flavonoid Glycosides from Ulmus macrocarpa Inhibit Osteoclast Differentiation via the Downregulation of NFATc1
Read this article at
Abstract
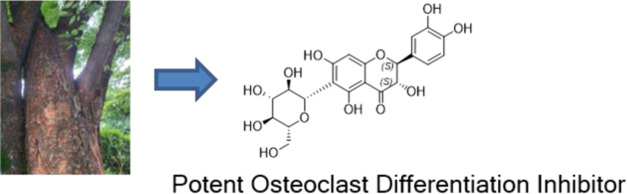
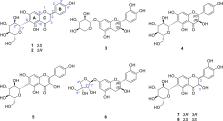
The aim of this study was to isolate and identify chemical components with osteoclast differentiation inhibitory activity from Ulmus macrocarpa Hance bark. Spectroscopic analyses, including nuclear magnetic resonance (NMR) and electronic circular dichroism (ECD), resulted in the unequivocal elucidation of active compounds such as (2 S)-naringenin-6- C-β- d-glucopyranoside ( 1), (2 R)-naringenin-6- C-β- d-glucopyranoside ( 2), (2 R,3 S)-catechin-7- O-β- d-xylopyranoside ( 3), (2 R,3 S)-catechin-7- O-β- d-apiofuranoside ( 6), (2 R,3 R)-taxifolin-6- C-β- d-glucopyranoside ( 7), and (2 S,3 S)-taxifolin-6- C-β- d-glucopyranoside ( 8). Mechanistically, the compounds may exhibit osteoclast differentiation inhibitory activity via the downregulation of NFATc1, a master regulator involved in osteoclast formation. This is the first report of their inhibitory activities on the receptor activator of nuclear factor κB ligand (RANKL)-induced osteoclast differentiation in murine bone marrow-derived macrophages. These findings provide further scientific evidence for the rational application of the genus Ulmus for the amelioration or treatment of osteopenic diseases.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Osteoclast differentiation and activation.
- Record: found
- Abstract: found
- Article: not found
Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems.
- Record: found
- Abstract: found
- Article: not found