- Record: found
- Abstract: found
- Article: found
Performance of an Automated Fluorescence Antinuclear Antibody Image Analyzer
Read this article at
Abstract
Background
The gold standard for antinuclear antibody (ANA) screening is the indirect immunofluorescence (IIF) assay with human epithelial cells (HEp-2). However, a number of substantial disadvantages of manual IIF assays have highlighted the need for the automation and standardization of fluorescent ANA (FANA) testing. We evaluated the performance of EUROPattern Suite (Euroimmun AG, Germany), an automated FANA image analyzer, with regard to ANA detection and pattern recognition compared with conventional manual interpretation using the fluorescence microscopic IIF assay.
Methods
A total of 104 samples including 70 ANA-positive sera and 34 ANA-negative sera collected from September to October 2015 were included. The sensitivity, specificity, and pattern recognition function were evaluated to determine the performance of EUROPattern Suite compared with the manual IIF assay results.
Results
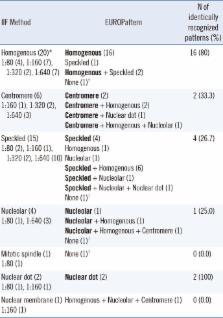
The sensitivity and specificity of EUROPattern Suite for ANA detection were 94.3% and 94.1%, respectively. The concordance rate between the two methods was 94.2%. For pattern recognition, 45.7% of the samples were assigned identical ANA patterns including simple and mixed. When major pattern matching was considered, 83.7% (41/49) and 95.2% (20/21) of the samples with simple and mixed patterns, respectively, showed concordant results between the two methods.
Conclusions
EUROPattern Suite, an automated FANA image analyzer, provides a viable option for distinguishing between positive and negative results, although the ability to assign specific patterns is insufficient to replace manual microscopic interpretation. This automated system may increase efficiency in laboratories, in which a large number of samples need to be processed.
Related collections
Most cited references16
- Record: found
- Abstract: found
- Article: not found
International consensus on ANA patterns (ICAP): the bumpy road towards a consensus on reporting ANA results
- Record: found
- Abstract: found
- Article: not found
Importance of the dense fine speckled pattern on HEp-2 cells and anti-DFS70 antibodies for the diagnosis of systemic autoimmune diseases.
- Record: found
- Abstract: found
- Article: not found