- Record: found
- Abstract: found
- Article: found
Evaluation of a Fully Automated Antinuclear Antibody Indirect Immunofluorescence Assay in Routine Use

Read this article at
Abstract
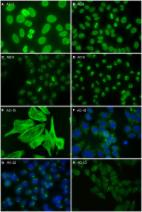
Indirect immunofluorescence assay (IFA) using HEp-2 cells as a substrate is the gold standard for detecting antinuclear antibodies (ANA) in patient serum. However, the ANA IFA has labor-intensive nature of the procedure and lacks adequate standardization. To overcome these drawbacks, the automation has been developed and implemented to the clinical laboratory. The purposes of this study were to evaluate the analytical performance of a fully automated Helios ANA IFA analyzer in a real-life laboratory setting, and to compare the time and the cost of ANA IFA testing before and after adopting the Helios system. A total of 3,276 consecutive serum samples were analyzed for ANA using the Helios system from May to August 2019. The positive/negative results, staining patterns, and endpoint titers were compared between Helios and visual readings. Furthermore, the turnaround time and the number of wells used were compared before and after the introduction of Helios system. Of the 3,276 samples tested, 748 were positive and 2,528 were negative based on visual readings. Using visual reading as the reference standard, the overall relative sensitivity, relative specificity, and concordance of Helios reading were 73.3, 99.4, and 93.4% ( κ = 0.80), respectively. For pattern recognition, the overall agreement was 70.1% (298/425) for single patterns, and 72.4% (89/123) for mixed patterns. For titration, there was an agreement of 75.9% (211/278) between automated and classical endpoint titers by regarding within ± one titer difference as acceptable. Helios significantly shortened the median turnaround time from 100.6 to 55.7 h ( P < 0.0001). Furthermore, routine use of the system reduced the average number of wells used per test from 4 to 1.5. Helios shows good agreement in distinguishing between positive and negative results. However, it still has limitations in positive/negative discrimination, pattern recognition, and endpoint titer prediction, requiring additional validation of results by human observers. Helios provides significant advantages in routine laboratory ANA IFA work in terms of labor, time, and cost savings. We hope that upgrading and developing softwares with more reliable capabilities will allow automated ANA IFA analyzers to be fully integrated into the routine operations of the clinical laboratory.
Related collections
Most cited references38

- Record: found
- Abstract: found
- Article: found
Interrater reliability: the kappa statistic
- Record: found
- Abstract: found
- Article: not found