- Record: found
- Abstract: found
- Article: found
Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance
Read this article at
Abstract
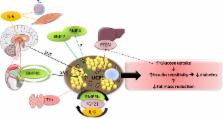
Presence of brown adipose tissue (BAT), characterized by the expression of the thermogenic uncoupling protein 1 (UCP1), has recently been described in adult humans. UCP1 is expressed in classical brown adipocytes, as well as in “beige cells” in white adipose tissue (WAT). The thermogenic activity of BAT is mainly controlled by the sympathetic nervous system. Endocrine factors, such as fibroblast growth factor 21 (FGF21) and bone morphogenic protein factor-9 (BMP-9), predominantly produced in the liver, were shown to lead to activation of BAT thermogenesis, as well as to “browning” of WAT. This was also observed in response to irisin, a hormone secreted by skeletal muscles. Different approaches were used to delineate the impact of UCP1 on insulin sensitivity. When studied under thermoneutral conditions, UCP1 knockout mice exhibited markedly increased metabolic efficiency due to impaired thermogenesis. The impact of UCP1 deletion on insulin sensitivity in these mice was not reported. Conversely, several studies in both rodents and humans have shown that BAT activation (by cold exposure, β3-agonist treatment, transplantation and others) improves glucose tolerance and insulin sensitivity. Interestingly, similar results were obtained by adipose tissue-specific overexpression of PR-domain-containing 16 (PRDM16) or BMP4 in mice. The mediators of such beneficial effects seem to include FGF21, interleukin-6, BMP8B and prostaglandin D2 synthase. Interestingly, some of these molecules can be secreted by BAT itself, indicating the occurrence of autocrine effects. Stimulation of BAT activity and/or recruitment of UCP1-positive cells are therefore relevant targets for the treatment of obesity/type 2 diabetes in humans.
Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: not found
New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure.
- Record: found
- Abstract: found
- Article: not found
Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships.
- Record: found
- Abstract: found
- Article: not found