- Record: found
- Abstract: found
- Article: found
CFTR Therapeutics Normalize Cerebral Perfusion Deficits in Mouse Models of Heart Failure and Subarachnoid Hemorrhage
Read this article at
Visual Abstract
Highlights
-
•
The cystic fibrosis transmembrane conductance regulator (CFTR) is a significant modulator of cerebrovascular reactivity; the loss of CFTR function enhances myogenic vasoconstriction.
-
•
Heart failure and subarachnoid hemorrhage downregulate cerebrovascular CFTR protein expression; this leads to enhanced cerebral artery vasoconstriction, reduced cerebral perfusion, neuronal injury, and ultimately, neurologic deficits.
-
•
CFTR therapeutics that maintain CFTR expression normalize the perfusion deficits, reduce neuronal injury, and improve neurologic function in these pathological settings.
Summary
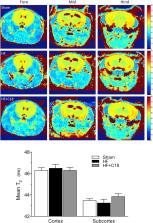
Heart failure (HF) and subarachnoid hemorrhage (SAH) chronically reduce cerebral perfusion, which negatively affects clinical outcome. This work demonstrates a strong relationship between cerebral artery cystic fibrosis transmembrane conductance regulator (CFTR) expression and altered cerebrovascular reactivity in HF and SAH. In HF and SAH, CFTR corrector compounds (C18 or lumacaftor) normalize pathological alterations in cerebral artery CFTR expression, vascular reactivity, and cerebral perfusion, without affecting systemic hemodynamic parameters. This normalization correlates with reduced neuronal injury. Therefore, CFTR therapeutics have emerged as valuable clinical tools to manage cerebrovascular dysfunction, impaired cerebral perfusion, and neuronal injury.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Novel Object Recognition Test for the Investigation of Learning and Memory in Mice
- Record: found
- Abstract: found
- Article: not found
Cerebral autoregulation.
- Record: found
- Abstract: found
- Article: not found
