- Record: found
- Abstract: not found
- Article: not found
Three structurally and functionally distinct β-glucuronidases from the human gut microbe Bacteroides uniformis
Samuel J. Pellock ,
William G. Walton ,
Kristen A. Biernat ,
Dariana Torres-Rivera ,
Benjamin C. Creekmore ,
Yongmei Xu ,
Jian Liu ,
Ashutosh Tripathy ,
Lance J. Stewart ,
Matthew R. Redinbo
November 30 2018
October 09 2018
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
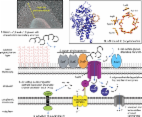
<p class="first" id="d558224e284">The glycoside hydrolases encoded by the human gut
microbiome play an integral role
in processing a variety of exogenous and endogenous glycoconjugates. Here we present
three structurally and functionally distinct β-glucuronidase (GUS) glycoside hydrolases
from a single human gut commensal microbe,
<i>Bacteroides uniformis</i>. We show using nine crystal structures, biochemical,
and biophysical data that whereas
these three proteins share similar overall folds, they exhibit different structural
features that create three structurally and functionally unique enzyme active sites.
Notably, quaternary structure plays an important role in creating distinct active
site features that are hard to predict via structural modeling methods. The enzymes
display differential processing capabilities toward glucuronic acid–containing polysaccharides
and SN-38-glucuronide, a metabolite of the cancer drug irinotecan. We also demonstrate
that GUS-specific and nonselective inhibitors exhibit varying potencies toward each
enzyme. Together, these data highlight the diversity of GUS enzymes within a single
<i>Bacteroides</i> gut commensal and advance our understanding of how structural details
impact the
specific roles microbial enzymes play in processing drug-glucuronide and glycan substrates.
</p>
Related collections
Most cited references18
- Record: found
- Abstract: not found
- Article: not found
Basic Local Alignment Search Tool
S Altschul (1990)
- Record: found
- Abstract: found
- Article: found
Complex Glycan Catabolism by the Human Gut Microbiota: The Bacteroidetes Sus-like Paradigm*
Eric C. Martens, Nicole M. Koropatkin, Thomas Smith … (2009)
- Record: found
- Abstract: found
- Article: not found
