- Record: found
- Abstract: found
- Article: found
Soman (GD) Rat Model to Mimic Civilian Exposure to Nerve Agent: Mortality, Video-EEG Based Status Epilepticus Severity, Sex Differences, Spontaneously Recurring Seizures, and Brain Pathology
Read this article at
Abstract
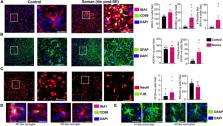
Modeling a real-world scenario of organophosphate nerve agent (OPNA) exposure is challenging. Military personnel are premedicated with pyridostigmine, which led to the development of OPNA models with pyridostigmine/oxime pretreatment to investigate novel therapeutics for acute and chronic effects. However, civilians are not premedicated with pyridostigmine/oxime. Therefore, experimental models without pyridostigmine were developed by other laboratories though often only in males. Following OPNA exposure, prolonged convulsive seizures (CS) or status epilepticus (SE) are concerning. The duration and severity of CS/SE determine the extent of brain injury in survivors even after treating with medical countermeasures (MCM)/antidotes such as atropine, an oxime, and an anticonvulsant such as diazepam/midazolam. In this study, using a large mixed sex cohort of adult male and female rats, without pretreatment, we demonstrate severe SE lasting for >20 min in 82% of the animals in response to soman (GD,132 μg/kg, s.c.). Atropine sulfate (2 mg/kg, i.m.) and HI-6 (125 mg/kg, i.m.) were administered immediately following soman, and midazolam (3 mg/kg, i.m.) 1 h post-exposure. Immediate MCM treatment is impractical in civilian exposure to civilians, but this approach reduces mortality in experimental models. Interestingly, female rats, irrespective of estrous stages, had an average of 44 min CS (stage ≥ 3), while males had an average of 32 min CS during SE, starting from soman exposure to midazolam treatment. However, in telemetry device implanted groups, there were no significant sex differences in SE severity; males had 40 min and females 43 min of continuous CS until midazolam was administered. No animals died prior to midazolam administration and less than 5% died in the first week after soman intoxication. In telemetered animals, there was a direct correlation between EEG changes and behavioral seizures in real-time. In the long-term, convulsive spontaneously recurring seizures (SRS) were observed in 85% of randomly chosen animals. At 4-months post-soman, the brain histology confirmed reactive gliosis and neurodegeneration. The novel findings of this study are that, in non-telemetered animals, the SE severity following soman intoxication was significantly greater in females compared to males and that the estrous cycle did not influence the response.
Related collections
Most cited references97
- Record: found
- Abstract: found
- Article: found
Acetylcholinesterase Inhibitors: Pharmacology and Toxicology

- Record: found
- Abstract: found
- Article: found