- Record: found
- Abstract: found
- Article: found
Disease-modifying effects of a glial-targeted inducible nitric oxide synthase inhibitor (1400W) in mixed-sex cohorts of a rat soman (GD) model of epilepsy
Read this article at
Abstract
Background
Acute exposure to seizurogenic organophosphate (OP) nerve agents (OPNA) such as diisopropylfluorophosphate (DFP) or soman (GD), at high concentrations, induce immediate status epilepticus (SE), reactive gliosis, neurodegeneration, and epileptogenesis as a consequence. Medical countermeasures (MCMs—atropine, oximes, benzodiazepines), if administered in < 20 min of OPNA exposure, can control acute symptoms and mortality. However, MCMs alone are inadequate to prevent OPNA-induced brain injury and behavioral dysfunction in survivors. We have previously shown that OPNA exposure-induced SE increases the production of inducible nitric oxide synthase (iNOS) in glial cells in both short- and long- terms. Treating with a water soluble and highly selective iNOS inhibitor, 1400W, for 3 days significantly reduced OPNA-induced brain changes in those animals that had mild–moderate SE in the rat DFP model. However, such mitigating effects and the mechanisms of 1400W are unknown in a highly volatile nerve agent GD exposure.
Methods
Mixed-sex cohort of adult Sprague Dawley rats were exposed to GD (132 μg/kg, s.c.) and immediately treated with atropine (2 mg/kg, i.m) and HI-6 (125 mg/kg, i.m.). Severity of seizures were quantified for an hour and treated with midazolam (3 mg/kg, i.m.). An hour post-midazolam, 1400W (20 mg/kg, i.m.) or vehicle was administered daily for 2 weeks. After behavioral testing and EEG acquisition, animals were euthanized at 3.5 months post-GD. Brains were processed for neuroinflammatory and neurodegeneration markers. Serum and CSF were used for nitrooxidative and proinflammatory cytokines assays.
Results
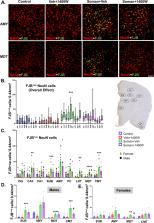
We demonstrate a significant long-term (3.5 months post-soman) disease-modifying effect of 1400W in animals that had severe SE for > 20 min of continuous convulsive seizures. 1400W significantly reduced GD-induced motor and cognitive dysfunction; nitrooxidative stress (nitrite, ROS; increased GSH: GSSG); proinflammatory cytokines in the serum and some in the cerebrospinal fluid (CSF); epileptiform spikes and spontaneously recurring seizures (SRS) in males; reactive gliosis (GFAP + C3 and IBA1 + CD68-positive glia) as a measure of neuroinflammation, and neurodegeneration (especially parvalbumin-positive neurons) in some brain regions.
Related collections
Most cited references82
- Record: found
- Abstract: found
- Article: not found
The classical complement cascade mediates CNS synapse elimination.
- Record: found
- Abstract: found
- Article: not found
PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel.
- Record: found
- Abstract: found
- Article: not found