- Record: found
- Abstract: found
- Article: found
Synthesis and Properties of Bis-Porphyrin Molecular Tweezers: Effects of Spacer Flexibility on Binding and Supramolecular Chirogenesis
Read this article at
Abstract
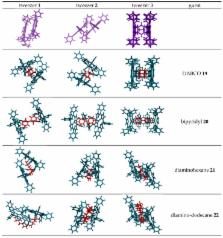
Ditopic binding of various dinitrogen compounds to three bisporphyrin molecular tweezers with spacers of varying conformational rigidity, incorporating the planar enediyne ( 1), the helical stiff stilbene ( 2), or the semi-rigid glycoluril motif fused to the porphyrins ( 3), are compared. Binding constants K a = 10 4–10 6 M −1 reveal subtle differences between these tweezers, that are discussed in terms of porphyrin dislocation modes. Exciton coupled circular dichroism (ECCD) of complexes with chiral dinitrogen guests provides experimental evidence for the conformational properties of the tweezers. The results are further supported and rationalized by conformational analysis.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Determining association constants from titration experiments in supramolecular chemistry.
- Record: found
- Abstract: found
- Article: not found
Application of electronic circular dichroism in configurational and conformational analysis of organic compounds.
- Record: found
- Abstract: not found
- Article: not found