- Record: found
- Abstract: found
- Article: found
Photoelectrochemical water oxidation by a MOF/semiconductor composite†

Read this article at
Abstract
Artificial photosynthesis is one of the most promising forms of renewable fuel production,
due to the abundance of water, carbon dioxide, and sunlight. However, the water oxidation
reaction remains a significant bottleneck due to the high thermodynamic and kinetic
requirements of the four-electron process. While significant work has been done on
the development of catalysts for water splitting, many of the catalysts reported to
date operate at high overpotentials or with the use of sacrificial oxidants to drive
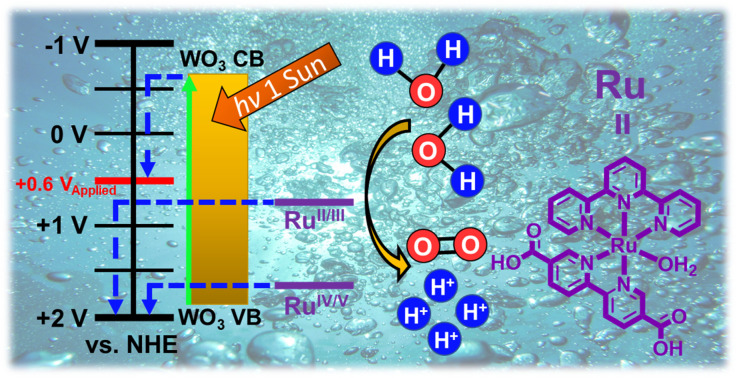
the reaction. Here, we present a catalyst embedded metal–organic framework (MOF)/semiconductor
composite that performs photoelectrochemical oxidation of water at a formal underpotential.
Ru-UiO-67 (where Ru stands for the water oxidation catalyst [Ru(tpy)(dcbpy)OH
2]
2+ (tpy = 2,2′:6′,2′′-terpyridine, dcbpy = 5,5-dicarboxy-2,2′-bipyridine)) has been
previously shown to be active for water oxidation under both chemical and electrochemical
conditions, but here we demonstrate, for the first time, incorporation of a light
harvesting n-type semiconductor as a base photoelectrode. Ru-UiO-67/WO
3 is active for photoelectrochemical water oxidation at a thermodynamic underpotential
(
η ≈ 200 mV;
E
onset = 600 mV
vs. NHE), and incorporation of a molecular catalyst onto the oxide layer increases efficiency
of charge transport and separation over bare WO
3. The charge-separation process was evaluated with ultrafast transient absorption
spectroscopy (ufTA) and photocurrent density measurements. These studies suggest that
a key contributor to the photocatalytic process involves a hole transfer from excited
 to Ru-UiO-67. To our knowledge, this is the first report of a MOF-based catalyst
active for water oxidation at a thermodynamic underpotential, a key step towards light-driven
water oxidation.
to Ru-UiO-67. To our knowledge, this is the first report of a MOF-based catalyst
active for water oxidation at a thermodynamic underpotential, a key step towards light-driven
water oxidation.
Abstract
Herein, we report the development of a MOF-semiconductor composite film active for water oxidation at a thermodynamic underpotential.
Related collections
Most cited references1
- Record: found
- Abstract: not found
- Book: not found