- Record: found
- Abstract: found
- Article: found
Adherens junction proteins on the move—From the membrane to the nucleus in intestinal diseases
Read this article at
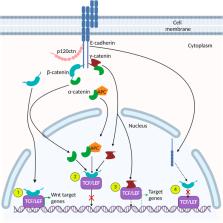
Abstract
The function and structure of the mammalian epithelial cell layer is maintained by distinct intercellular adhesion complexes including adherens junctions (AJs), tight junctions, and desmosomes. The AJ is most integral for stabilizing cell-cell adhesion and conserving the structural integrity of epithelial tissues. AJs are comprised of the transmembrane protein E-cadherin and cytoplasmic catenin cofactors (α, β, γ, and p120-catenin). One organ where malfunction of AJ is a major contributor to disease states is the mammalian intestine. In the intestine, cell-cell adhesion complexes work synergistically to maintain structural integrity and homeostasis of the epithelium and prevent its malfunction. Consequently, when AJ integrity is compromised in the intestinal epithelium, the ensuing homeostatic disruption leads to diseases such as inflammatory bowel disease and colorectal carcinoma. In addition to their function at the plasma membrane, protein components of AJs also have nuclear functions and are thus implicated in regulating gene expression and intracellular signaling. Within the nucleus, AJ proteins have been shown to interact with transcription factors such as TCF/LEF and Kaiso ( ZBTB33), which converge on the canonical Wnt signaling pathway. The multifaceted nature of AJ proteins highlights their complexity in modulating homeostasis and emphasizes the importance of their subcellular localization and expression in the mammalian intestine. In this review, we summarize the nuclear roles of AJ proteins in intestinal tissues; their interactions with transcription factors and how this leads to crosstalk with canonical Wnt signaling; and how nuclear AJ proteins are implicated in intestinal homeostasis and disease.
Related collections
Most cited references165
- Record: found
- Abstract: found
- Article: not found
Intestinal epithelial cells: regulators of barrier function and immune homeostasis.
- Record: found
- Abstract: found
- Article: not found
The many faces and functions of β-catenin.
- Record: found
- Abstract: found
- Article: not found