- Record: found
- Abstract: found
- Article: found
Interferon-Alpha Promotes Th1 Response and Epithelial Apoptosis via Inflammasome Activation in Human Intestinal Mucosa

Read this article at
Abstract
Backgound & Aims
Several lines of investigation suggest that interferon (IFN) alpha can alter human intestinal mucosa homeostasis. These include the endogenous production of IFN alpha in celiac disease or inflammatory bowel diseases, as well as the occurrence of intestinal side effects of exogenous IFN alpha used as a therapeutic tool. Here, we present an ex vivo translational approach to investigate the effects of IFN alpha on the human normal intestinal mucosa, as well as its underlying mechanisms.
Methods
Human normal colonic mucosa explants were cultured in the presence or absence of IFN alpha 2a. Epithelial homeostasis was assessed using the immunohistochemical marker of apoptosis M30. The Wnt inhibitor Dickkopf-Homolog-1 (DKK1) was assayed in the supernatants by enzyme-linked immunosorbent assay. Activation of the inflammasome (caspase-1/interleukin [IL]18) and of a Th1 response was determined by in situ detection of active caspase-1, as well as by measurement of mature IL18 production and the prototype Th1 cytokine IFN gamma by enzyme-linked immunosorbent assay. In addition, mechanistic studies were performed using the specific caspase-1 inhibitor Tyr-Val-Ala-Asp(OMe)-fluoromethylketone (YVAD-FMK), IL18-binding protein, neutralizing anti–IFN gamma, and anti-DKK1 antibodies.
Results
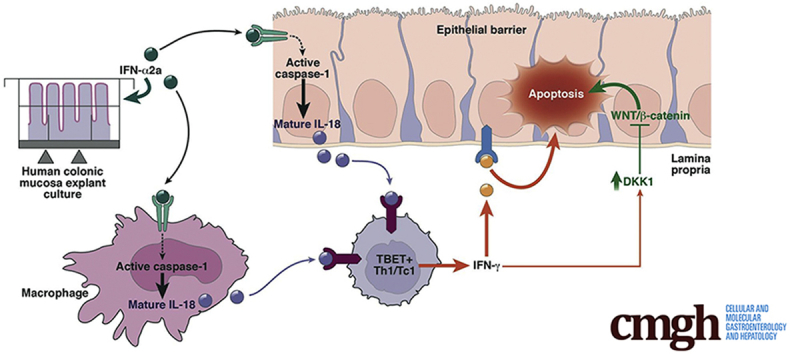
IFN alpha 2a elicited a rapid (24 hours) disruption of surface and crypt colonic epithelial cells via apoptosis that was variable in intensity among the 20 individuals studied. This apoptotic effect was dependent on the initiation of an IFN gamma response elicited by resident T box expressed in T cells–positive lamina propria cells. Both apoptosis and Th1 response were subordinated to active caspase-1 and IL18 production. Finally, neutralization of IFN gamma–induced DKK1 partially protected against IFN alpha–induced epithelial apoptosis.
Conclusions
By using an ex vivo model, we show an interindividual heterogeneity of IFN alpha effects. We show that IFN alpha is able to disrupt both epithelial and immune homeostasis in the human intestine, by activation of an innate immunity platform, the inflammasome, which drives a Th1 response and leads to epithelial barrier disruption.
Graphical abstract
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
The inflammasomes: guardians of the body.
- Record: found
- Abstract: found
- Article: not found
Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms.
- Record: found
- Abstract: found
- Article: not found