- Record: found
- Abstract: found
- Article: found
Shaped 3D microcarriers for adherent cell culture and analysis
Read this article at
Abstract
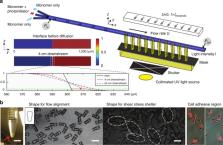
Standard tissue culture of adherent cells is known to poorly replicate physiology and often entails suspending cells in solution for analysis and sorting, which modulates protein expression and eliminates intercellular connections. To allow adherent culture and processing in flow, we present 3D-shaped hydrogel cell microcarriers, which are designed with a recessed nook in a first dimension to provide a tunable shear-stress shelter for cell growth, and a dumbbell shape in an orthogonal direction to allow for self-alignment in a confined flow, important for processing in flow and imaging flow cytometry. We designed a method to rapidly design, using the genetic algorithm, and manufacture the microcarriers at scale using a transient liquid molding optofluidic approach. The ability to precisely engineer the microcarriers solves fundamental challenges with shear-stress-induced cell damage during liquid-handling, and is poised to enable adherent cell culture, in-flow analysis, and sorting in a single format.
Adherent cells: microcarriers for flow cytometry
A new microcarrier for adherent cells is demonstrated which allows for accurate flow cytometry and high-speed imaging without risk of flow-induced damage. Microcarriers are attractive for accelerated cell culture, passaging and analysis, but they must be designed to promote cell growth and analysis without flow-induced cell damage. A team led by Dino Di Carlo at University of California, Los Angeles now report a 3D-shaped microparticle that features a region of extracellular matrix for cell adhesion and culture physically protected from shear flow. Key to the design is the intersection of two 2D patterns, leading to a shape which can align with flow inside the channel during cytometry, and also provides a cut-away region to protect cells during culture. These microcarriers may facilitate high-speed adherent cell screening for applications such as drug discovery.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Generation of human induced pluripotent stem cells from dermal fibroblasts.
- Record: found
- Abstract: found
- Article: not found
Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs.
- Record: found
- Abstract: found
- Article: not found