- Record: found
- Abstract: found
- Article: found
HAb18G/CD147 cell-cell contacts confer resistance of a HEK293 subpopulation to anoikis in an E-cadherin-dependent manner
Read this article at
Abstract
Background
Acquisition of resistance to "anoikis" facilitates the survival of cells under independent matrix-deficient conditions, such as cells in tumor progression and the production of suspension culture cells for biomedical engineering. There is evidence suggesting that CD147, an adhesion molecule associated with survival of cells in tumor metastasis and cell-cell contacts, plays an important role in resistance to anoikis. However, information regarding the functions of CD147 in mediating cell-cell contacts and anoikis-resistance remains limited and even self-contradictory.
Results
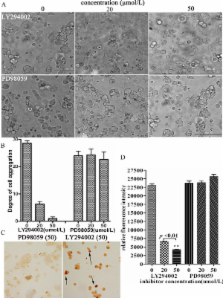
An anoikis-resistant clone (HEK293ar), derived from anoikis-sensitive parental Human Embryonic Kidney 293 cells, survived anoikis by the formation of cell-cell contacts. The expression of HAb18G/CD147 (a member of the CD147 family) was upregulated and the protein was located at cell-cell junctions. Upregulation of HAb18G/CD147 in suspended HEK293ar cells suppressed anoikis by mediating the formation of cell-cell adhesions. Anoikis resistance in HEK293ar cells also required E-cadherin-mediated cell-cell contacts. Knock-down of HAb18G/CD147 and E-cadherin inhibited cell-cell contacts formation and increased anoikis sensitivity respectively. When HAb18G/CD147 was downregulated, E-cadherin expression in HEK293ar cells was significantly suppressed; however, knockdown of E-cadherin by E-cadherin siRNA or blocking of E-cadherin binding activity with a specific antibody and EDTA had no significant effect on HAb18G/CD147 expression. Finally, pretreatment with LY294002, a phosphoinositide 3-kinase (PI3K/AKT) inhibitor, disrupted cell-cell contacts and decreased cell number, but this was not the case in cells treated with the extracellular signal-regulated kinase (ERK) inhibitor PD98059.
Conclusions
Our results provide new evidence that HAb18G/CD147-mediated cell-cell contact confers anoikis resistance in an E-cadherin-dependent manner; and cell-cell contact mediated resistance to anoikis implicates PI3K pathway in a highly relevant cell model (HEK293ar). Understanding of the role of HAb18G/CD147 cell-cell contacts in anoikis resistance may help in understanding the survival of cells in anchorage-independent growth, such as cells in tumor metastasis and suspension culture produced for biomedical engineering. Our results also contribute to a better understanding of the biology of HEK293 cell spheroids, a major workhorse for producing human therapeutic agents and viral vaccines.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Anoikis resistance and tumor metastasis.
- Record: found
- Abstract: found
- Article: not found
Molecular mechanisms of "detachment-induced apoptosis--Anoikis".
- Record: found
- Abstract: found
- Article: not found