- Record: found
- Abstract: found
- Article: found
Schiff-Linked PEGylated Doxorubicin Prodrug Forming pH-Responsive Nanoparticles With High Drug Loading and Effective Anticancer Therapy
Read this article at
Abstract
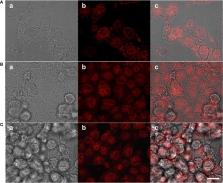
Developing efficacious drug delivery systems for targeted cancer chemotherapy remains a major challenge. Here we demonstrated a kind of pH-responsive PEGylated doxorubicin (DOX) prodrug via the effective esterification and Schiff base reactions, which could self-assemble into the biodegradable micelles in aqueous solutions. Owing to low pH values inside the tumor cells, these PEG-Schiff-DOX nanoparticles exhibited high drug loading ability and pH-responsive drug release behavior within the tumor cells or tissues upon changes in physical and chemical environments, but they displayed good stability at physiological conditions for a long period. CCK-8 assay showed that these PEGylated DOX prodrugs had a similar cytotoxicity to the MCF-7 tumor cells as the free DOX drug. Moreover, this kind of nanoparticle could also encapsulate small DOX drugs with high drug loading, sufficient drug release and enhanced therapeutic effects toward MCF-7 cells, which will be benefited for developing more drug carriers with desirable functions for clinical anticancer therapy.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Multidrug resistance in cancer: role of ATP-dependent transporters.
- Record: found
- Abstract: found
- Article: not found
Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity.
- Record: found
- Abstract: found
- Article: not found