- Record: found
- Abstract: found
- Article: found
A comparison of the temporary placement of 3 different self-expanding stents for the treatment of refractory benign esophageal strictures: a prospective multicentre study
Read this article at
Abstract
Background
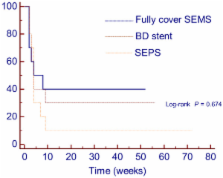
Refractory benign esophageal strictures (RBESs) have been treated with the temporary placement of different self-expanding stents with conflicting results. We compared the clinical effectiveness of 3 types of stents: self-expanding plastic stents (SEPSs), biodegradable stents, and fully covered self-expanding metal stents (FCSEMSs), for the treatment of RBES.
Methods
This study prospectively evaluated 3 groups of 30 consecutive patients with RBESs who underwent temporary placement of either SEPSs (12 weeks, n = 10), biodegradable stents (n = 10) or FCSEMSs (12 weeks, n = 10). Data were collected to analyze the technical success and clinical outcome of the stents as evaluated by recurrent dysphagia, complications and reinterventions.
Results
Stent implantation was technically successful in all patients. Migration occurred in 11 patients: 6 (60%) in the SEPS group, 2 (20%) in the biodegradable group and 3 (30%) in the FCSEMS group ( P = 0.16). A total of 8/30 patients (26.6%) were dysphagia-free after the end of follow-up: 1 (10%) in the SEPS group, 3 (30%) in the biodegradable group and 4 (40%) in the FCSEMS group ( P = 0.27). More reinterventions were required in the SEPS group (n = 24) than in the biodegradable group (n = 13) or the FCSEMS group (n = 13) ( P = 0.24). Multivariate analysis showed that stricture length was significantly associated with higher recurrence rates after temporary stent placement (HR = 1.37; 95% CI = 1.08-1.75; P = 0.011).
Conclusions
Temporary placement of a biodegradable stent or of a FCSEMS in patients with RBES may lead to long-term relief of dysphagia in 30 and 40% of patients, respectively. The use of SEPSs seems least preferable, as they are associated with frequent stent migration, more reinterventions and few cases of long-term improvement. Additionally, longer strictures were associated with a higher risk of recurrence.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer.
- Record: found
- Abstract: found
- Article: not found
Role of esophageal stents in benign and malignant diseases.
- Record: found
- Abstract: found
- Article: not found