- Record: found
- Abstract: found
- Article: found
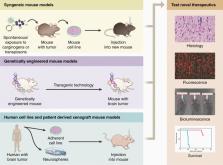
Mouse models of glioblastoma for the evaluation of novel therapeutic strategies
Read this article at
Abstract
Glioblastoma (GBM) is an incurable brain tumor with a median survival of approximately 15 months despite an aggressive standard of care that includes surgery, chemotherapy, and ionizing radiation. Mouse models have advanced our understanding of GBM biology and the development of novel therapeutic strategies for GBM patients. However, model selection is crucial when testing developmental therapeutics, and each mouse model of GBM has unique advantages and disadvantages that can influence the validity and translatability of experimental results. To shed light on this process, we discuss the strengths and limitations of 3 types of mouse GBM models in this review: syngeneic models, genetically engineered mouse models, and xenograft models, including traditional xenograft cell lines and patient-derived xenograft models.
Related collections
Most cited references157
- Record: found
- Abstract: found
- Article: not found
Tumor mutational load predicts survival after immunotherapy across multiple cancer types
- Record: found
- Abstract: found
- Article: found
Comprehensive genomic characterization defines human glioblastoma genes and core pathways
- Record: found
- Abstract: found
- Article: not found