- Record: found
- Abstract: found
- Article: found
Copper Ions and Parkinson’s Disease: Why Is Homeostasis So Relevant?
Read this article at
Abstract
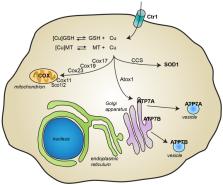
The involvement of copper in numerous physiological processes makes this metal ion essential for human life. Alterations in copper homeostasis might have deleterious consequences, and several neurodegenerative disorders, including Parkinson’s disease (PD), have been associated with impaired copper levels. In the present review, we describe the molecular mechanisms through which copper can exert its toxicity, by considering how it can interfere with other cellular processes known to play a role in PD, such as dopamine metabolism, oxidative stress, and α-synuclein aggregation. The recent experimental evidence that associates copper deficiency and the formation of superoxide dismutase 1 (SOD1) aggregates with the progression of PD is also discussed together with its therapeutic implication. Overall, the recent discoveries described in this review show how either copper deficiency or excessive levels can promote detrimental effects, highlighting the importance of preserving copper homeostasis and opening unexplored therapeutic avenues in the definition of novel disease-modifying drugs.
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
Targeting chelatable iron as a therapeutic modality in Parkinson's disease.
- Record: found
- Abstract: found
- Article: not found
Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease.
- Record: found
- Abstract: found
- Article: not found