- Record: found
- Abstract: found
- Article: found
A Dhdds K42E knock-in RP59 mouse model shows inner retina pathology and defective synaptic transmission
Read this article at
Abstract
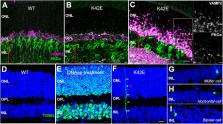
Retinitis pigmentosa (RP) defines a group of hereditary progressive rod-cone degenerations that exhibit a common phenotype caused by variants in over 70 genes. While most variants in the dehydro dolichyl diphosphate synthase (DHDDS) gene result in syndromic abnormalities, some variants cause non-syndromic RP (RP59). DHDDS encodes one subunit of the enzyme cis-prenyltransferase (CPT), which is required for the synthesis of dolichol (Dol), that is a necessary protein glycosylation cofactor. We previously reported the creation and initial characterization of a knock-in (KI) mouse model harboring the most prevalent RP59-associated DHDDS variant (K42E) to understand how defects in DHDDS lead to retina-specific pathology. This model exhibited no profound retinal degeneration, nor protein N-glycosylation defects. Here, we report that the Dol isoprenylogue species in retina, liver, and brain of the K42E mouse model are statistically shorter than in the corresponding tissues of age-matched controls, as reported in blood and urine of RP59 patients. Retinal transcriptome analysis demonstrated elevation of many genes encoding proteins involved in synaptogenesis and synaptic function. Quantitative retinal cell layer thickness measurements demonstrated a significant reduction in the inner nuclear layer (INL) and total retinal thickness (TRT) beginning at postnatal (PN) ∼2 months, progressively increasing to PN 18-mo. Histological analysis revealed cell loss in the INL, outer plexiform layer (OPL) disruption, and ectopic localization of outer nuclear layer (ONL) nuclei into the OPL of K42E mutant retinas, relative to controls. Electroretinograms (ERGs) of mutant mice exhibited reduced b-wave amplitudes beginning at PN 1-mo, progressively declining through PN 18-mo, without appreciable a-wave attenuation, relative to controls. Our results suggest that the underlying cause of DHDDS K42E variant driven RP59 retinal pathology is defective synaptic transmission from outer to inner retina.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system.
- Record: found
- Abstract: found
- Article: not found
Retinal remodeling during retinal degeneration.
- Record: found
- Abstract: found
- Article: not found