- Record: found
- Abstract: not found
- Article: not found
Personalized noninvasive respiratory support for acute hypoxemic respiratory failure
editorial
28 April 2023
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Physiological rationale
The last decade has witnessed major changes in the management of patients with acute
de novo hypoxemic respiratory failure (AHRF). Noninvasive support may help avoid endotracheal
intubation and reduce the detrimental effects of sedation and invasive mechanical
ventilation. However, if noninvasive support fails and the patient is subsequently
intubated (30–60% of cases), it can lead to increased mortality [1]. A more thorough
understanding of the physiology of spontaneous breathing has led a new bedside conundrum:
meticulously balancing the use of noninvasive devices to avoid intubation against
the risk of exposure to delayed intubation combined to the newly described concept
of self-inflicted lung injury (P-SILI). P-SILI occurs due to increased inspiratory
effort (ΔPES) and trans-pulmonary pressures swings leading inhomogeneous lung inflation
and local overstretch. Despite the existence of P-SILI remains debated, this physiologic
concept may plausibly explain the adverse events noted in some patients failing noninvasive
support.
In addition to standard oxygen therapy, high-flow nasal cannula (HFNC), continuous
positive airway pressure (CPAP), and pressure-support noninvasive ventilation (NIV)
are the most extensively applied noninvasive respiratory support modalities in the
acute care setting. The latter two modalities may be delivered by either a facemask
or a helmet interface. The different devices have varying mechanisms that may impact
oxygenation, ventilation, and cardiac physiology in patients with AHRF [2]. Below
we summarize the most recent evidence for noninvasive respiratory support in de novo
AHRF. The evidence summarized does not focus on the application post-extubation, on
post-operative patients, respiratory failure due to cardiogenic pulmonary edema and
acute exacerbation of chronic pulmonary diseases.
Clinical evidence
A series of meta-analyses have demonstrated a potential role of facemask NIV/CPAP
in preventing intubation compared to standard oxygen therapy. These studies focused
predominantly on AHRF of low severity and have not shown a consistent benefit [3].
Enthusiasm for facemask NIV was challenged by the results of the FLORALI trial, which
suggested a possible higher risk of intubation and mortality in the most severely
ill patients (PaO2/FiO2 < 200 mmHg) treated with NIV compared to HFNC [4]. Randomized
trials of HFNC versus standard oxygen therapy have consistently shown reduced need
for endotracheal intubation [5], but no effect on mortality [6, 7].
During the coronavirus disease 2019 (COVID-19) pandemic, a trial by Perkins and coworkers
found that an initial strategy of CPAP, mostly delivered through a facemask with low
levels of PEEP (8 cmH2O), reduced a composite endpoint of endotracheal intubation
or mortality within 30 days, compared to standard oxygen therapy [8]. Because of some
limitations including early termination, a high frequency of crossover between the
treatments and the lack of prespecified criteria for intubation, the results of this
trial warrant further confirmatory investigations.
In a single-center exploratory trial, Patel and coworkers found lower rates of intubation
and mortality with helmet compared to facemask NIV; the observed huge mortality benefit
in the intervention group can be considered as surprising, especially because of the
limited sample and the early termination of enrollment [9]. However, these intriguing
findings led to further evaluation into the mechanisms by which the helmet interface
could optimize the support of AHRF patients. These mechanisms include effective administration
of higher PEEP (> 10 cmH2O), which may improve oxygenation and mitigate the risk of
self-inflicted lung injury by inspiratory effort modulation and more homogeneous lung
inflation [2]. The first head-to-head comparison of helmet NIV alternating with HFNC
compared to HFNC alone in patients with COVID-19 and PaO2/FiO2 < 200 mmHg (HENIVOT)
found, as an exploratory outcome, lower rates of intubation in patients treated with
the helmet, who received a median PEEP of 12 cmH2O. However, a subsequent larger trial
comparing helmet NIV to usual respiratory support (standard oxygen, facemask NIV or
HFNC) did not find differences in intubation rate or mortality [10].
Taken together, these data highlight the uncertainty about the optimal initial strategy
for noninvasive support in patients AHRF. Currently, due to its simplicity and reduction
in intubation compared to standard oxygen therapy, HFNC represents the suggested initial
treatment of AHRF by clinical guidelines [5]. High PEEP delivered through the helmet
interface represents the most promising alternative strategy; ongoing clinical trials
are evaluating this approach.
Physiologically based patient-targeted strategies
Emerging concepts around noninvasive devices have highlighted that (1) a one-size-fits-all
approach is likely not beneficial in AHRF and (2) delayed intubation after prolonged
exposure to spontaneous breathing may be associated with worse outcome. Given this,
while studies surrounding individualized treatments of AHRF are underway, available
tools capable of assessing the risk of self-inflicted lung injury and early identification
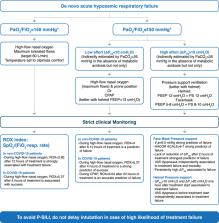
of treatment failure are essential for clinicians at the bedside (Fig. 1).
Fig. 1
Example of algorithm (authors’ viewpoint) for the initial management and monitoring
of patients with de novo acute hypoxemic respiratory failure with no preexisting cardiac
or pulmonary diseases or metabolic acidosis/alkalosis. This approach is specifically
intended for de novo acute hypoxemic respiratory failure and is not intended for acute
respiratory failure due to cardiogenic pulmonary edema and post-operative respiratory
failure. *For patients undergoing standard oxygen through a non-rebreathing facemask,
FiO2 can be estimated as: 21% + oxygen flow rate in L/min × 3. Acronyms. PEEP positive
end-expiratory pressure. PS pressure support. ΔP
ES
esophageal pressure inspiratory swing. V
T
e expired tidal volume. P-SILI patient self-inflicted lung injury, VAS visual analog
scale, HACOR composite scale including heart rate, acidosis, consciousness, oxygenation
and respiratory rate
From a physiological standpoint, AHRF is characterized by different degrees of hypoxemia
combined or not with intense inspiratory effort [1]. Convincing evidence indicates
that HFNC represents the most effective tool to treat patients with mild-to-moderate
hypoxemia (PaO2/FiO2 > 150 mmHg) [5, 7]. For patients with moderate-to-severe hypoxemia
(PaO2/FiO2 < 150 mmHg), in whom facemask NIV may potentially yield poor outcomes,
it is uncertain whether a strategy of early CPAP (facemask or helmet), helmet NIV
or either strategy combined with HFNC sessions may provide clinical benefit over HFNC
alone. Recent data indicate that patients’ phenotyping based on the intensity of inspiratory
effort may aid clinical decision. A physiological study on AHRF patients with PaO2/FiO2 < 200 mmHg
showed that high-PEEP helmet NIV and CPAP are both capable of improving oxygenation,
compared to HFNC. NIV can decreases inspiratory effort by unloading the respiratory
muscles but may increase trans-pulmonary driving pressure, especially in patients
who have low inspiratory effort (< 10 cmH2O) during HFNC. Conversely, CPAP does not
usually affect inspiratory effort and does not increase trans-pulmonary driving pressure
[2].
Importantly, in patients with AHRF and no metabolic acidosis, the intensity of inspiratory
effort may be associated to hypocapnia. Accordingly, in a post hoc analysis of the
HENIVOT trial, helmet NIV was shown to reduce the rate of endotracheal intubation
and improve survival as compared to HFNC in patients with PaCO2 < 35 mmHg. Helmet
NIV had no effect on outcome in normocapnic patients (PaCO2 > 35 mmHg) [11]. These
data may suggest that patients with high effort (ΔPES > 10 cmH2O with high tidal volumes
and minute ventilation and thus PaCO2 < 35 mmHg) may benefit from NIV. Patients with
low effort (ΔPES < 10 cmH2O and/or PaCO2 > 35 mmHg) may be better treated with HFNC
or CPAP delivered with either face mask or helmet, eventually combined with awake
prone position to improve oxygenation [12, 13]. This observation however does not
exclude that some patients with normal PaCO2 could exhibit high effort (in case of,
for instance, increased dead space/poor ventilation perfusion ratio) and thus benefit
from NIV.
New technological developments to non-invasively assess inspiratory effort at the
bedside are mandatory to allow us to optimize the use of the noninvasive techniques
for respiratory support. Promising techniques may include diaphragmatic ultrasound
and transcutaneous measurement of diaphragmatic activity.
Identification of treatment failure
During any treatment, the lack of improvement in gas exchange, persistent signs of
respiratory distress and increased work of breathing (tachypnea and dyspnea) should
indicate the need for endotracheal intubation and a high risk of P-SILI. Importantly,
when PEEP is applied, the initial improvement in oxygenation should not be seen as
conclusive sign of treatment success, as it may be falsely reassuring.
During HFNC, the ratio of SpO2/FiO2 to respiratory rate (ROX index) < 3.85 after 12 h
of treatment and a worsening ROX index over time have been associated with increased
risk of subsequent intubation [14]. During CPAP, there is a paucity of validated tools
to identify treatment failure at an early time point. Recently, ROX index < 6 at 24 h
has been shown to predict the need for endotracheal intubation [15]. During NIV, tidal
volume > 9.5 ml/kg of predicted body weight and persistently high inspiratory effort
(ΔPES > 10 cmH2O) are parameters strongly associated to subsequent failure. Unfortunately,
tidal volume measurement may be difficult in the presence of leaks during facemask
NIV and is impossible with conventional tools during helmet support. Similarly, inspiratory
effort assessment currently requires esophageal manometry, which is not always feasible
in non-intubated patients. Studies to identify noninvasive surrogates for inspiratory
effort are ongoing. A composite scale (HACOR) including heart rate, acidosis, consciousness,
oxygenation, and respiratory rate has been shown to accurately identify patients prone
to treatment failure 6 h after NIV initiation [16], and may aid clinical decision-making.
Related collections
Most cited references16
- Record: found
- Abstract: found
- Article: not found
High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure.
Jean-Pierre Frat, Arnaud W Thille, Alain Mercat … (2015)
- Record: found
- Abstract: found
- Article: not found
An Index Combining Respiratory Rate and Oxygenation to Predict Outcome of Nasal High-Flow Therapy
Oriol Roca, Berta Caralt, Jonathan Messika … (2019)
- Record: found
- Abstract: found
- Article: not found
Effect of Noninvasive Ventilation Delivered by Helmet vs Face Mask on the Rate of Endotracheal Intubation in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial.
Bhakti K Patel, Krysta S Wolfe, Anne Pohlman … (2016)
