- Record: found
- Abstract: found
- Article: not found
Mcp5, a meiotic cell cortex protein, is required for nuclear movement mediated by dynein and microtubules in fission yeast
Read this article at
Abstract
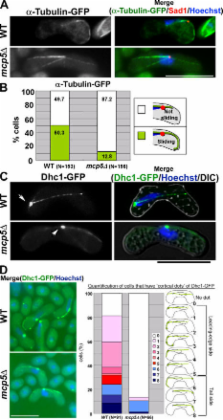
During meiotic prophase I of the fission yeast Schizosaccharomyces pombe, oscillatory nuclear movement occurs. This promotes homologous chromosome pairing and recombination and involves cortical dynein, which plays a pivotal role by generating a pulling force with the help of an unknown dynein anchor. We show that Mcp5, the homologue of the budding yeast dynein anchor Num1, may be this putative dynein anchor. mcp5 + is predominantly expressed during meiotic prophase, and GFP-Mcp5 localizes at the cell cortex. Moreover, the mcp5Δ strain lacks the oscillatory nuclear movement. Accordingly, homologous pairing and recombination rates of the mcp5Δ strain are significantly reduced. Furthermore, the cortical localization of dynein heavy chain 1 appears to be reduced in mcp5Δ cells. Finally, the full function of Mcp5 requires its coiled-coil and pleckstrin homology (PH) domains. Our results suggest that Mcp5 localizes at the cell cortex through its PH domain and functions as a dynein anchor, thereby facilitating nuclear oscillation.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
RADIOAUTOGRAPHIC STUDIES OF CHOLINE INCORPORATION INTO PERIPHERAL NERVE MYELIN
- Record: found
- Abstract: found
- Article: not found
Telomere-led premeiotic chromosome movement in fission yeast.
- Record: found
- Abstract: found
- Article: not found
