- Record: found
- Abstract: found
- Article: found
IGF-1C domain–modified hydrogel enhanced the efficacy of stem cells in the treatment of AMI
Read this article at
Abstract
Background
Due to the low survival rate of cell transplantation, stem cell has not been widely used in clinical treatment of acute myocardial infarction (AMI). In this study, we immobilized the C domain peptide of insulin-like growth factor-1 on chitosan (CS-IGF-1C) to obtain bioactive hydrogel. The purpose was to investigate whether CS-IGF-1C hydrogel incorporated with human placenta–derived mesenchymal stem cells (hP-MSCs) can boost the survival of hP-MSCs and enhance their therapeutic effects.
Methods
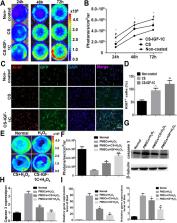
hP-MSCs, which continuously expressed green fluorescent protein (GFP) and firefly luciferase (Fluc), were transplanted with CS-IGF-1C hydrogel into a mouse myocardial infarction model. Cell survival was detected by bioluminescence imaging (BLI), and cardiac function was measured by echocardiogram. Real-time PCR and histological analysis were used to explore the therapeutic mechanism of CS-IGF-1C hydrogel.
Results
CS-IGF-1C hydrogel could induce the proliferation of hP-MSCs and exert anti-apoptotic effects in vitro. The Calcine-AM/PI staining results showed that hP-MSCs seeded on CS-IGF-1C hydrogel could protect neonatal mouse ventricular cardiomyocytes (NMVCs) against oxidative stress. It was observed by BLI that CS-IGF-1C hydrogel injected into ischemic myocardium could improve the survival rate of hP-MSCs. Histology analysis indicated that co-transplantation of the CS-IGF-1C hydrogel and hP-MSCs could increase angiogenesis, reduce collagen deposition, ameliorate left ventricular expanded, and further promote the recovery of cardiac function. Besides, we found that the inflammatory response was inhibited and the expression of apoptosis-related genes was downregulated by CS-IGF-1C hydrogel.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse.
- Record: found
- Abstract: found
- Article: not found
IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair.
- Record: found
- Abstract: found
- Article: not found