- Record: found
- Abstract: found
- Article: found
Recent Progress on Polysaccharide-Based Hydrogels for Controlled Delivery of Therapeutic Biomolecules

Read this article at
Abstract
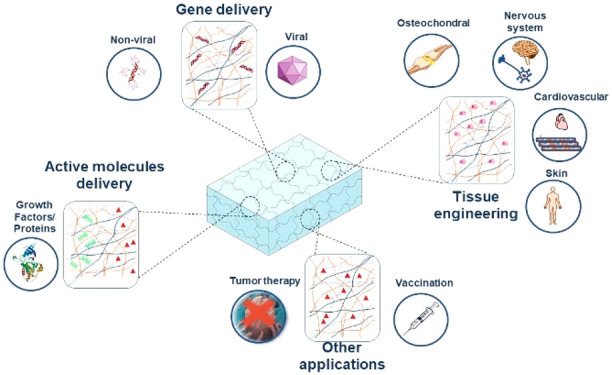
A plethora of applications using polysaccharides have been developed in recent years due to their availability as well as their frequent nontoxicity and biodegradability. These polymers are usually obtained from renewable sources or are byproducts of industrial processes, thus, their use is collaborative in waste management and shows promise for an enhanced sustainable circular economy. Regarding the development of novel delivery systems for biotherapeutics, the potential of polysaccharides is attractive for the previously mentioned properties and also for the possibility of chemical modification of their structures, their ability to form matrixes of diverse architectures and mechanical properties, as well as for their ability to maintain bioactivity following incorporation of the biomolecules into the matrix. Biotherapeutics, such as proteins, growth factors, gene vectors, enzymes, hormones, DNA/RNA, and antibodies are currently in use as major therapeutics in a wide range of pathologies. In the present review, we summarize recent progress in the development of polysaccharide-based hydrogels of diverse nature, alone or in combination with other polymers or drug delivery systems, which have been implemented in the delivery of biotherapeutics in the pharmaceutical and biomedical fields.
Related collections
Most cited references293
- Record: found
- Abstract: found
- Article: not found
Designing hydrogels for controlled drug delivery
- Record: found
- Abstract: found
- Article: not found
Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody
- Record: found
- Abstract: found
- Article: not found