- Record: found
- Abstract: found
- Article: not found
Novel bis-amide-based bis-thiazoles as Anti-colorectal Cancer Agents Through Bcl-2 Inhibition: Synthesis, In Vitro, and In Vivo studies
Read this article at
Abstract
Background:
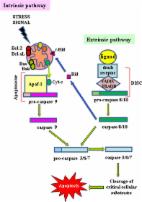
Some heterocycles having bisamide linkage are receiving much interest due to their remarkable biological potencies and they are naturally occurring. Some bisamides and thiazole derivatives were found to inhibit the protein levels of Bcl-2 significantly. This prompted us to synthesize new bis(heterocyclic) derivatives having bisamide function to explore their anti-cancer activities.
Method:
Novel bis-amide-based bis-thiazoles and thiadiazoles were synthesized by reaction of a new bisthiosemicarbazone with a variety of hydrazonoyl chlorides, a-chloroacetylacetone and haloacetic acid derivatives. Most of the synthesized derivatives were tested for colorectal (HCT-116) and breast (MCF-7) cell lines using the MTT assay, with the apoptotic investigation through flow cytometric and RT-PCR analyses.
Results:
Some derivatives were found to be highly cytotoxic against HCT-116 cells with an IC50 range of (10.44-13.76 μM) compared to 5-fluorouracil (5-FU) (IC50 = 11.78 μM). One product significantly stimulated apoptotic colorectal cancer cell death by 27.24-fold (50.13% compared to control 1.84%) by arresting the cell cycle at the G2/M phase. The obtained results revealed that compound 7f was more cytotoxic against HCT-116 cells than 5-FU. Compound 7f remarkably enhanced apoptotic colorectal cancer cell death and upregulated the propapoptotic genes (P53, BAX and Capases-3,-8,-9) and downregulated the anti-apoptotic gene, B-cell lymphoma 2 (Bcl-2). In vivo study exhibited that 7f-treatment caused tumor inhibition ratio (TIR%) of 50.45% compared to 54.86% in the 5-FU treatment, with a significant reduction in tumor mass and volume. The anti-tumor activity of compound 7f was accompanied by ameliorated hematological and biochemical analyses, histopathological improvement in treated liver tissues, and the immunohistochemical staining revealed Bcl-2 inhibition in agreement with the in vitro results.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays
- Record: found
- Abstract: found
- Article: found