- Record: found
- Abstract: found
- Article: found
Propranolol for Treatment of Infantile Hemangioma: Efficacy and Effect on Pediatric Growth and Development
Read this article at
Abstract
Purpose
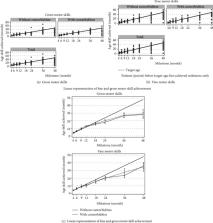
Propranolol has been successful in treating problematic infantile hemangiomas (IH) but concerns regarding its effect on normal growth and development have been raised. This study examines physical growth, developmental milestones, and human growth hormone (hGH) levels in infants receiving propranolol for problematic IH.
Method
Monthly heights and weights of children undergoing propranolol therapy for IH were prospectively collected and tabulated. Data analysis and comparison to World Health Organization (WHO) weight-for-age and weight-to-length z-scores was performed. Questionnaires regarding milestones, efficacy, and guardian satisfaction were performed, and a combination of both chart results and phone conducted surveys was tabulated. Serum from a small representative cohort of age-matched children with IH treated and not treated with propranolol was collected.
Results
A total of 185 children receiving propranolol therapy between 2008 and 2013 for IH were assigned to this study. The children were divided into two cohorts based on the presence of comorbidities or risk factors that may affect growth and development ( n = 142 no comorbidities, n = 43 with comorbidities). Neither cohort demonstrated deviation from normal weight in comparison to WHO normative data. There was a significant deviation for BMI-for-age and weight-for-age z-scores in our population, especially in patients on propranolol for more than 7 months. Based on data from participants, via either completed questionnaires or chart results, most children met their developmental milestones at or before target ages, regardless of the presence of comorbidities. Eighty percent of guardians noticed clinical improvement of the IH, with 91% either happy about or neutral to using the medication. hGH levels were higher in patients receiving propranolol therapy, but not significantly different.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
A randomized, controlled trial of oral propranolol in infantile hemangioma.
- Record: found
- Abstract: not found
- Article: not found
Propranolol for severe hemangiomas of infancy.
- Record: found
- Abstract: found
- Article: not found