- Record: found
- Abstract: found
- Article: found
A Global Collaboration to Develop and Pilot Test a Mobile Application to Improve Cancer Pain Management in Nepal

Read this article at
Abstract
Introduction
Quality palliative care, which prioritizes comfort and symptom control, can reduce global suffering from non-communicable diseases, such as cancer. To address this need, the Nepalese Association of Palliative Care (NAPCare) created pain management guidelines (PMG) to support healthcare providers in assessing and treating serious pain. The NAPCare PMG are grounded in World Health Organization best practices but adapted for the cultural and resource context of Nepal. Wider adoption of the NAPCare PMG has been limited due to distribution of the guidelines as paper booklets.
Methods
Building on a long-standing partnership between clinicians and researchers in the US and Nepal, the NAPCare PMG mobile application (“app”) was collaboratively designed. Healthcare providers in Nepal were recruited to pilot test the app using patient case studies. Then, participants completed a Qualtrics survey to evaluate the app which included the System Usability Scale (SUS) and selected items from the Mobile App Rating Scale (MARS). Descriptive and summary statistics were calculated and compared across institutions and roles. Regression analyses to explore relationships (α = 0.05) between selected demographic variables and SUS and MARS scores were also conducted.
Results
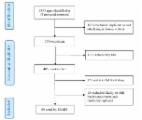
Ninety eight healthcare providers ( n = 98) pilot tested the NAPCare PMG app. Overall, across institutions and roles, the app received an SUS score of 76.0 (a score > 68 is considered above average) and a MARS score of 4.10 (on a scale of 1 = poor, 5 = excellent). 89.8% ( n = 88) “agreed” or “strongly agreed” that the app will help them better manage cancer pain. Age, years of experience, and training in palliative care were significant in predicting SUS scores ( p-values, 0.0124, 0.0371, and 0.0189, respectively); institution was significant in predicting MARS scores ( p = 0.0030).
Conclusion
The NAPCare PMG mobile app was well-received, and participants rated it highly on both the SUS and MARS. Regression analyses suggest end-user variables important to consider in designing and evaluating mobile apps in lower resourced settings. Our app design and pilot testing process illustrate the benefits of cross global collaborations to build research capacity and generate knowledge within the local context.
Related collections
Most cited references76
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries
- Record: found
- Abstract: not found
- Article: not found
Sample size of 12 per group rule of thumb for a pilot study
- Record: found
- Abstract: found
- Article: found