- Record: found
- Abstract: found
- Article: found
A Modular Approach for the Synthesis of Diverse Heterobifunctional Cyanine Dyes
research-article
27 February 2024
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract

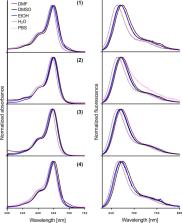
Herein, we present a straightforward synthetic route for the design and synthesis of diverse heterobifunctional cyanine 5 dyes. We optimized the workup by harnessing the pH- and functional group-dependent solubility of the asymmetric cyanine 5 dyes. Therefore, purification through chromatography is deferred until the last synthesis step. Demonstrating successful large-scale synthesis, our modular approach prevents functional group degradation by introducing them in the last synthesis step. These modifiable heterobifunctional dyes offer significant utility in advancing biological studies.
Related collections
Most cited references55
- Record: found
- Abstract: not found
- Article: not found
Simple Method for the Esterification of Carboxylic Acids
Bernhard Neises, Wolfgang Steglich (1978)
- Record: found
- Abstract: found
- Article: not found
A review of NIR dyes in cancer targeting and imaging.
Shenglin Luo, Erlong Zhang, Yongping Su … (2011)
- Record: found
- Abstract: found
- Article: not found
Targeted protein degradation by PROTACs.
Taavi Neklesa, James D Winkler, Craig M Crews (2017)