- Record: found
- Abstract: found
- Article: found
Metal-catalyzed uncaging of DNA-binding agents in living cells†

Read this article at
Abstract
Abstract
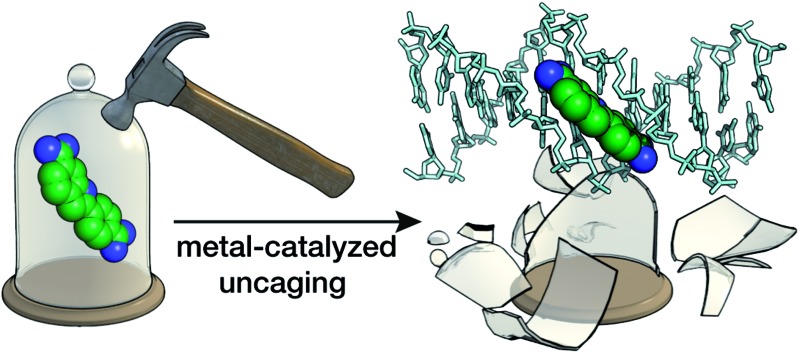
Attachment of alloc protecting groups to the amidine units of fluorogenic DNA-binding bisbenzamidines or to the amino groups of ethidium bromide leads to a significant reduction of their DNA affinity. More importantly, the active DNA-binding species can be readily regenerated by treatment with ruthenium catalysts in aqueous conditions, even in cell cultures. The catalytic chemical uncaging can be easily monitored by fluorescence microscopy, because the protected products display both different emission properties and cell distribution to the parent compounds.
Related collections
Most cited references18
- Record: found
- Abstract: not found
- Article: not found
Complex formation between ethidium bromide and nucleic acids.
- Record: found
- Abstract: found
- Article: not found
Antiparasitic compounds that target DNA.
- Record: found
- Abstract: found
- Article: not found
Organometallic compounds: an opportunity for chemical biology?
Author and article information
Notes
†Electronic supplementary information (ESI) available: Synthesis and characterization of the studied molecules and required precursors. NMR, UV, and fluorescence spectra, titrations, control experiments, and detailed procedures for cell uptake and co-staining experiments. See DOI: 10.1039/c3sc53317d