- Record: found
- Abstract: found
- Article: found
A cell-penetrating artificial metalloenzyme regulates a gene switch in a designer mammalian cell
Read this article at
Abstract
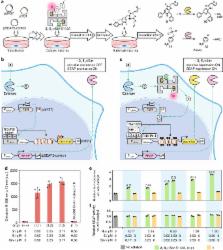
Complementing enzymes in their native environment with either homogeneous or heterogeneous catalysts is challenging due to the sea of functionalities present within a cell. To supplement these efforts, artificial metalloenzymes are drawing attention as they combine attractive features of both homogeneous catalysts and enzymes. Herein we show that such hybrid catalysts consisting of a metal cofactor, a cell-penetrating module, and a protein scaffold are taken up into HEK-293T cells where they catalyze the uncaging of a hormone. This bioorthogonal reaction causes the upregulation of a gene circuit, which in turn leads to the expression of a nanoluc-luciferase. Relying on the biotin–streptavidin technology, variation of the biotinylated ruthenium complex: the biotinylated cell-penetrating poly(disulfide) ratio can be combined with point mutations on streptavidin to optimize the catalytic uncaging of an allyl-carbamate-protected thyroid hormone triiodothyronine. These results demonstrate that artificial metalloenzymes offer highly modular tools to perform bioorthogonal catalysis in live HEK cells.
Abstract
Artificial enzymes can be used to elicit reactions in cells. Here, the authors developed such an artificial catalyst combined with a genetic switch, and showed that it was readily taken up by human cells and able to kick off a reaction cascade resulting in the biosynthesis of the desired product.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Artificial Metalloenzymes: Reaction Scope and Optimization Strategies.
- Record: found
- Abstract: found
- Article: not found