- Record: found
- Abstract: found
- Article: found
Fe 3O 4-Au Core-Shell Nanoparticles as a Multimodal Platform for In Vivo Imaging and Focused Photothermal Therapy
Read this article at
Abstract
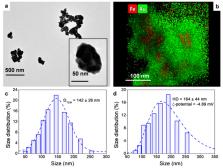
In this study, we report the synthesis of gold-coated iron oxide nanoparticles capped with polyvinylpyrrolidone (Fe@Au NPs). The as-synthesized nanoparticles (NPs) exhibited good stability in aqueous media and excellent features as contrast agents (CA) for both magnetic resonance imaging (MRI) and X-ray computed tomography (CT). Additionally, due to the presence of the local surface plasmon resonances of gold, the NPs showed exploitable “light-to-heat” conversion ability in the near-infrared (NIR) region, a key attribute for effective photothermal therapies (PTT). In vitro experiments revealed biocompatibility as well as excellent efficiency in killing glioblastoma cells via PTT. The in vivo nontoxicity of the NPs was demonstrated using zebrafish embryos as an intermediate step between cells and rodent models. To warrant that an effective therapeutic dose was achieved inside the tumor, both intratumoral and intravenous routes were screened in rodent models by MRI and CT. The pharmacokinetics and biodistribution confirmed the multimodal imaging CA capabilities of the Fe@AuNPs and revealed constraints of the intravenous route for tumor targeting, dictating intratumoral administration for therapeutic applications. Finally, Fe@Au NPs were successfully used for an in vivo proof of concept of imaging-guided focused PTT against glioblastoma multiforme in a mouse model.
Related collections
Most cited references83

- Record: found
- Abstract: found
- Article: found
Combination therapy in combating cancer
- Record: found
- Abstract: found
- Article: not found
Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells.
- Record: found
- Abstract: found
- Article: not found