- Record: found
- Abstract: found
- Article: found
The Golgi Apparatus of Neocortical Glial Cells During Hibernation in the Syrian Hamster
Read this article at
Abstract
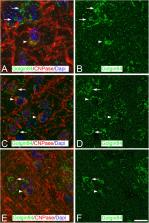
Hibernating mammals undergo torpor periods characterized by a general decrease in body temperature, metabolic rate, and brain activity accompanied by complex adaptive brain changes that appear to protect the brain from extreme conditions of hypoxia and low temperatures. These processes are accompanied by morphological and neurochemical changes in the brain including those in cortical neurons such as the fragmentation and reduction of the Golgi apparatus (GA), which both reverse a few hours after arousal from the torpor state. In the present study, we characterized – by immunofluorescence and confocal microscopy – the GA of cortical astrocytes, oligodendrocytes, and microglial cells in the Syrian hamster, which is a facultative hibernator. We also show that after artificial induction of hibernation, in addition to neurons, the GA of glia in the Syrian hamster undergoes important structural changes, as well as modifications in the intensity of immunostaining and distribution patterns of Golgi structural proteins at different stages of the hibernation cycle.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
Characterization of a cis-Golgi matrix protein, GM130
- Record: found
- Abstract: found
- Article: not found