- Record: found
- Abstract: found
- Article: found
Effect of Phosphorylated Tau on Cortical Pyramidal Neuron Morphology during Hibernation
Read this article at
Abstract
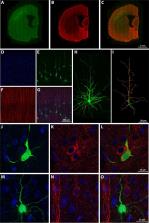
The dendritic spines of pyramidal cells are the main postsynaptic target of excitatory glutamatergic synapses. Morphological alterations have been described in hippocampal dendritic spines during hibernation—a state of inactivity and metabolic depression that occurs via a transient neuronal tau hyperphosphorylation. Here, we have used the hibernating Syrian hamster to investigate the effect of hyperphosphorylated tau regarding neocortical neuronal structure. In particular, we examined layer Va pyramidal neurons. Our results indicate that hibernation does not promote significant changes in dendritic spine density. However, tau hyperphosphorylated neurons show a decrease in complexity, an increase in the tortuosity of the apical dendrites, and an increase in the diameter of the basal dendrites. Tau protein hyperphosphorylation and aggregation have been associated with loss or alterations of dendritic spines in neurodegenerative diseases, such as Alzheimer’s disease (AD). Our results may shed light on the correlation between tau hyperphosphorylation and the neuropathological processes in AD. Moreover, we observed changes in the length and area of the apical and basal dendritic spines during hibernation regardless of tau hyperphosphorylation. The morphological changes observed here also suggest region specificity, opening up debate about a possible relationship with the differential brain activity registered in these regions in previous studies.
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature.
- Record: found
- Abstract: found
- Article: not found
Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model.
- Record: found
- Abstract: found
- Article: not found