- Record: found
- Abstract: found
- Article: found
Adenovirus-Mediated CCR7 and BTLA Overexpression Enhances Immune Tolerance and Migration in Immature Dendritic Cells
Read this article at
Abstract
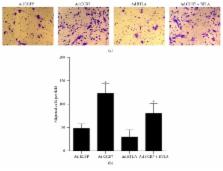
Our previous report revealed that immature dendritic cells (imDCs) with adenovirus-mediated CCR7 overexpression acquired an enhanced migratory ability but also exhibited the lower immune tolerance observed in more mature cells. In the present study, we aimed to investigate whether BTLA overexpression was sufficient to preserve immune tolerance in imDCs with exogenous CCR7 overexpression. Scanning electron microscopy and surface antigens analysis revealed that BTLA overexpression suppressed DC maturation, an effect further potentiated in CCR7 and BTLA cooverexpressing cells. Correspondingly, in vitro chemotaxis assays and mixed lymphocyte reactions demonstrated increased migratory potential and immune tolerance in CCR7 and BTLA coexpressing cells. Furthermore, CCR7 and BTLA cooverexpressed imDCs suppressed IFN- γ and IL-17 expression and promoted IL-4 and TGF-beta expression of lymphocyte, indicating an increase of T helper 2 (Th2) regulatory T cell (Treg). Thus, these data indicate that CCR7 and BTLA cooverexpression imparts an intermediate immune phenotype in imDCs when compared to that in CCR7- or BTLA-expressing counterparts that show a more immunocompetent or immunotolerant phenotype, respectively. All these results indicated that adenovirus-mediated CCR7 and BTLA overexpression could enhance immune tolerance and migration of imDCs. Our study provides a basis for further studies on imDCs in immune tolerance, with the goal of developing effective cellular immunotherapies for transplant recipients.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions.
- Record: found
- Abstract: found
- Article: not found
B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator.
- Record: found
- Abstract: found
- Article: not found