- Record: found
- Abstract: found
- Article: found
The Origins and Vulnerabilities of Two Transmissible Cancers in Tasmanian Devils

Read this article at
Summary
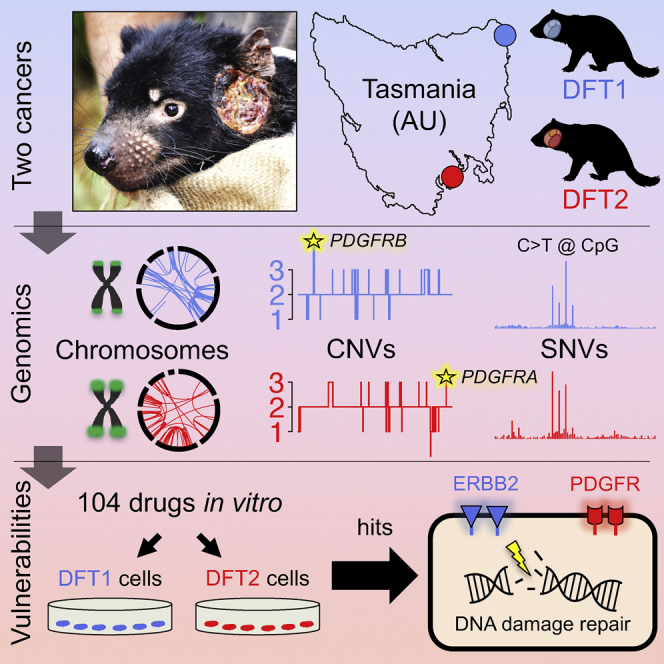
Transmissible cancers are clonal lineages that spread through populations via contagious cancer cells. Although rare in nature, two facial tumor clones affect Tasmanian devils. Here we perform comparative genetic and functional characterization of these lineages. The two cancers have similar patterns of mutation and show no evidence of exposure to exogenous mutagens or viruses. Genes encoding PDGF receptors have copy number gains and are present on extrachromosomal double minutes. Drug screening indicates causative roles for receptor tyrosine kinases and sensitivity to inhibitors of DNA repair. Y chromosome loss from a male clone infecting a female host suggests immunoediting. These results imply that Tasmanian devils may have inherent susceptibility to transmissible cancers and present a suite of therapeutic compounds for use in conservation.
Graphical Abstract
Highlights
-
•
Tasmanian devil transmissible cancers arose from similar tissues in two individuals
-
•
Similar mutation patterns and driver candidates imply common oncogenic processes
-
•
Losses at B2M and Y chromosome loci suggest selection to escape immune detection
-
•
Receptor tyrosine kinases and DNA repair factors implicated as therapeutic targets
Abstract
Stammnitz et al. show that the two transmissible cancer clones that affect Tasmanian devils are very similar in their tissues-of-origin, mutational patterns and driver gene candidates. Importantly, these cancers are both highly sensitive to inhibitors of some receptor tyrosine kinases as well as to inhibitors of DNA repair.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Targeting EZH2 in cancer.
- Record: found
- Abstract: found
- Article: not found
Timing, rates and spectra of human germline mutation
- Record: found
- Abstract: found
- Article: not found