- Record: found
- Abstract: found
- Article: found
Characterizing the role of rice NRAMP5 in Manganese, Iron and Cadmium Transport
Read this article at
Abstract
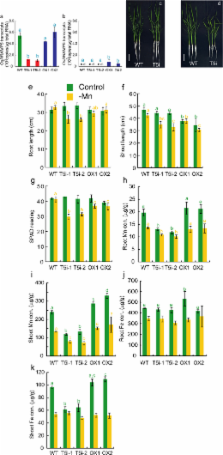
Metals like manganese (Mn) and iron (Fe) are essential for metabolism, while cadmium (Cd) is toxic for virtually all living organisms. Understanding the transport of these metals is important for breeding better crops. We have identified that OsNRAMP5 contributes to Mn, Fe and Cd transport in rice. OsNRAMP5 expression was restricted to roots epidermis, exodermis, and outer layers of the cortex as well as in tissues around the xylem. OsNRAMP5 localized to the plasma membrane, and complemented the growth of yeast strains defective in Mn, Fe, and Cd transport. OsNRAMP5 RNAi (OsNRAMP5i) plants accumulated less Mn in the roots, and less Mn and Fe in shoots, and xylem sap. The suppression of OsNRAMP5 promoted Cd translocation to shoots, highlighting the importance of this gene for Cd phytoremediation. These data reveal that OsNRAMP5 contributes to Mn, Cd, and Fe transport in rice and is important for plant growth and development.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth.
- Record: found
- Abstract: found
- Article: not found
Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+.
- Record: found
- Abstract: found
- Article: not found