- Record: found
- Abstract: found
- Article: found
Role of vascular smooth muscle cell clonality in atherosclerosis
Read this article at
Abstract
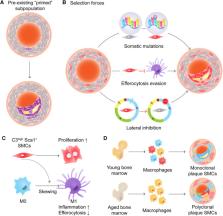
Atherosclerotic cardiovascular disease remains the leading cause of death worldwide. While many cell types contribute to the growing atherosclerotic plaque, the vascular smooth muscle cell (SMC) is a major contributor due in part to its remarkable plasticity and ability to undergo phenotype switching in response to injury. SMCs can migrate into the fibrous cap, presumably stabilizing the plaque, or accumulate within the lesional core, possibly accelerating vascular inflammation. How SMCs expand and react to disease stimuli has been a controversial topic for many decades. While early studies relying on X-chromosome inactivation were inconclusive due to low resolution and sensitivity, recent advances in multi-color lineage tracing models have revitalized the concept that SMCs likely expand in an oligoclonal fashion during atherogenesis. Current efforts are focused on determining whether all SMCs have equal capacity for clonal expansion or if a “stem-like” progenitor cell may exist, and to understand how constituents of the clone decide which phenotype they will ultimately adopt as the disease progresses. Mechanistic studies are also beginning to dissect the processes which confer cells with their overall survival advantage, test whether these properties are attributable to intrinsic features of the expanding clone, and define the role of cross-talk between proliferating SMCs and other plaque constituents such as neighboring macrophages. In this review, we aim to summarize the historical perspectives on SMC clonality, highlight unanswered questions, and identify translational issues which may need to be considered as therapeutics directed against SMC clonality are developed as a novel approach to targeting atherosclerosis.
Related collections
Most cited references88
- Record: found
- Abstract: found
- Article: not found
The clonal evolution of tumor cell populations.
- Record: found
- Abstract: found
- Article: found
Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future
- Record: found
- Abstract: found
- Article: not found