- Record: found
- Abstract: found
- Article: found
Plasma Microbial Cell-Free DNA Sequencing in Immunocompromised Patients With Pneumonia: A Prospective Observational Study

Read this article at
Abstract
Background
Pneumonia is a common cause of morbidity and mortality, yet a causative pathogen is identified in a minority of cases. Plasma microbial cell-free DNA sequencing may improve diagnostic yield in immunocompromised patients with pneumonia.
Methods
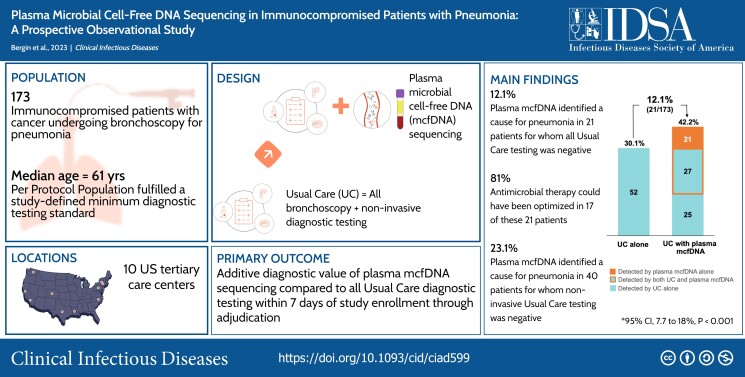
In this prospective, multicenter, observational study of immunocompromised adults undergoing bronchoscopy to establish a pneumonia etiology, plasma microbial cell-free DNA sequencing was compared to standardized usual care testing. Pneumonia etiology was adjudicated by a blinded independent committee. The primary outcome, additive diagnostic value, was assessed in the Per Protocol population (patients with complete testing results and no major protocol deviations) and defined as the percent of patients with an etiology of pneumonia exclusively identified by plasma microbial cell-free DNA sequencing. Clinical additive diagnostic value was assessed in the Per Protocol subgroup with negative usual care testing.
Results
Of 257 patients, 173 met Per Protocol criteria. A pneumonia etiology was identified by usual care in 52/173 (30.1%), plasma microbial cell-free DNA sequencing in 49/173 (28.3%) and the combination of both in 73/173 (42.2%) patients. Plasma microbial cell-free DNA sequencing exclusively identified an etiology of pneumonia in 21/173 patients (additive diagnostic value 12.1%, 95% confidence interval [CI], 7.7% to 18.0%, P < .001). In the Per Protocol subgroup with negative usual care testing, plasma microbial cell-free DNA sequencing identified a pneumonia etiology in 21/121 patients (clinical additive diagnostic value 17.4%, 95% CI, 11.1% to 25.3%).
Abstract
In this prospective multicenter observational study of immunocompromised patients undergoing bronchoscopy, plasma microbial cell-free DNA sequencing exclusively identified an adjudicated pneumonia etiology in 12.1% of patients with otherwise nondiagnostic testing, significantly increasing overall diagnostic yield in this high-risk population.
Graphical Abstract
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/plasma-microbial-cell-free-dna-sequencing-in-immunocompromised-patients-with-pneumonia-aprospective-observational-study-bb13c9de-87ac-430c-a84e-6fe9f3f79932
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group.
- Record: found
- Abstract: not found
- Article: not found
Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease
- Record: found
- Abstract: found
- Article: not found