- Record: found
- Abstract: found
- Article: found
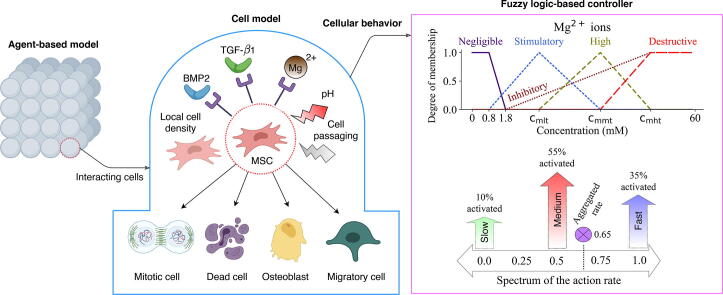
Magnesium ions regulate mesenchymal stem cells population and osteogenic differentiation: A fuzzy agent-based modeling approach

Read this article at
Graphical abstract
Abstract
Mesenchymal stem cells (MSCs) are proliferative and multipotent cells that play a key role in the bone regeneration process. Empirical data have repeatedly shown the bioregulatory importance of magnesium (Mg) ions in MSC growth and osteogenesis. In this study, we propose an agent-based model to predict the spatiotemporal dynamics of the MSC population and osteogenic differentiation in response to Mg 2+ ions. A fuzzy-logic controller was designed to govern the decision-making process of cells by predicting four cellular processes of proliferation, differentiation, migration, and mortality in response to several important bioregulatory factors such as Mg 2+ ions, pH, BMP2, and TGF-β1. The model was calibrated using the empirical data obtained from three sets of cell culture experiments. The model successfully reproduced the empirical observations regarding live cell count, viability, DNA content, and the differentiation-related markers of alkaline phosphate (ALP) and osteocalcin (OC). The simulation results, in agreement with the empirical data, showed that Mg 2+ ions within 3–6 mM concentration have the highest stimulation effect on cell population growth. The model also correctly reproduced the stimulatory effect of Mg 2+ ions on ALP and its inhibitory effect on OC as the early and late differentiation markers, respectively. Besides, the numerical simulation shed light on the innate cellular differences of the cells cultured in different experiments in terms of the proliferative capacity as well as sensitivity to Mg 2+ ions. The proposed model can be adopted in the study of the osteogenesis around Mg-based implants where ions released due to degradation interact with local cells and regulate bone regeneration.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro.

- Record: found
- Abstract: found
- Article: found
Bone biomaterials and interactions with stem cells
- Record: found
- Abstract: found
- Article: not found