- Record: found
- Abstract: found
- Article: found
The osteogenetic activities of mesenchymal stem cells in response to Mg 2+ ions and inflammatory cytokines: a numerical approach using fuzzy logic controllers
Read this article at
Abstract
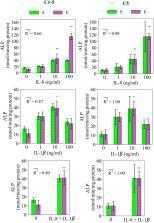
Magnesium (Mg 2+) ions are frequently reported to regulate osteogenic activities of mesenchymal stem cells (MSCs). In this study, we propose a numerical model to study the regulatory importance of Mg 2+ ions on MSCs osteoblastic differentiation in the presence of an inflammatory response. A fuzzy logic controller was formulated to receive the concentrations of Mg 2+ ions and the inflammatory cytokines of TNF-α, IL-10, IL-1β, and IL-8 as cellular inputs and predict the cells’ early and late differentiation rates. Five sets of empirical data obtained from published cell culture experiments were used to calibrate the model. The model successfully reproduced the empirical data regarding the concentration- and phase-dependent effect of Mg 2+ ions on the differentiation process. In agreement with the experiments, the model showed the stimulatory role of Mg 2+ ions on the early differentiation phase, once administered at low concentration, and their inhibitory role on the late differentiation phase. The numerical approach used in this study suggested 6–8 mM as the most effective concentration of Mg 2+ ions in promoting the early differentiation process. Also, the proposed model sheds light on the fundamental differences in the behavioral properties of cells cultured in different experiments, e.g. differentiation rate and the sensitivity of the cultured cells to stimulatory signals such as Mg 2+ ions. Thus, it can be used to interpret and compare different empirical findings. Moreover, the model successfully reproduced the nonlinearities in the concentration-dependent role of the inflammatory cytokines in early and late differentiation rates. Overall, the proposed model can be employed in studying the osteogenic properties of Mg-based implants in the presence of an inflammatory response.
Author summary
Magnesium (Mg) is an attractive material for bone implants as it fully degrades after implantation, saving pain and cost of the second surgery for implant removal. To advance its application in the orthopedic industry, it is paramount to fully understand the biological impact of the degradation products, in particular Mg 2+ ions. Here, we propose a computer model to study the effects of Mg 2+ ions on bone regeneration. The model focuses on stem cells and includes both the direct stimulation effects of Mg 2+ ions on cells and the indirect stimulus through the inflammatory system. The proposed model successfully reproduced the experimental data of five different studies. The model additionally highlighted differences amongst different experiments in terms of the cellular response to Mg 2+ ions. The proposed system therefore provides an important addition to the field of Mg implant research.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis.

- Record: found
- Abstract: found
- Article: found
The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine
- Record: found
- Abstract: found
- Article: not found